The impact of agarose immobilization on the activity of lytic Pseudomonas aeruginosa phages combined with chemicals
- PMID: 36625915
- PMCID: PMC9842590
- DOI: 10.1007/s00253-022-12349-4
The impact of agarose immobilization on the activity of lytic Pseudomonas aeruginosa phages combined with chemicals
Abstract
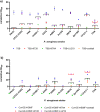
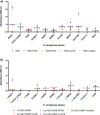
The implementation of non-traditional antibacterials is currently one of the most intensively explored areas of modern medical and biological sciences. One of the most promising alternative strategies to combat bacterial infections is the application of lytic phages combined with established and new antibacterials. The presented study investigates the potential of agarose-based biocomposites containing lytic Pseudomonas phages (KT28, KTN4, and LUZ19), cupric ions (Cu2+), strawberry furanone (HDMF), and gentamicin (GE) as antibacterials and anti-virulent compounds for novel wound dressings. Phages (KT28, KTN4, LUZ19, and triple-phage cocktail) alone and in combination with a triple-chemical mixture (Cu + GE + HDMF) when applied as the liquid formulation caused a significant bacterial count reduction and biofilm production inhibition of clinical P. aeruginosa strains. The immobilization in the agarose scaffold significantly impaired the bioavailability and diffusion of phage particles, depending on virion morphology and targeted receptor specificity. The antibacterial potential of chemicals was also reduced by the agarose scaffold. Moreover, the Cu + GE + HDMF mixture impaired the lytic activity of phages depending on viral particles' susceptibility to cupric ion toxicity. Therefore, three administration types were tested and the optimal turned out to be the one separating antibacterials both physically and temporally. Taken together, the additive effect of phages combined with chemicals makes biocomposite a good solution for designing new wound dressings. Nevertheless, the phage utilization should involve an application of aqueous cocktails directly onto the wound, followed by chemicals immobilized in hydrogel dressings which allow for taking advantage of the antibacterial and anti-virulent effects of all components. KEY POINTS: • The immobilization in the agarose impairs the bioavailability of phage particles and the Cu + GE + HDMF mixture. • The cupric ions are toxic to phages and are sequestrated on phage particles and agarose matrix. • The elaborated TIME-SHIFT administration effectively separates antibacterials both physically and temporally.
Keywords: Agarose immobilization; Cupric ions; Gentamicin; HDMF furanone; Phages; Pseudomonas aeruginosa.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Arabski M, Wasik S, Dworecki K, Kaca W. Laser interferometric determination of ampicillin and colistin transfer through cellulose biomembrane in the presence of Proteus vulgaris O25 lipopolysaccharide. J Memb Sci. 2007;299:268–275. doi: 10.1016/j.memsci.2007.05.003. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

