Stakeholder Perspectives of Clinical Artificial Intelligence Implementation: Systematic Review of Qualitative Evidence
- PMID: 36626192
- PMCID: PMC9875023
- DOI: 10.2196/39742
Stakeholder Perspectives of Clinical Artificial Intelligence Implementation: Systematic Review of Qualitative Evidence
Abstract
Background: The rhetoric surrounding clinical artificial intelligence (AI) often exaggerates its effect on real-world care. Limited understanding of the factors that influence its implementation can perpetuate this.
Objective: In this qualitative systematic review, we aimed to identify key stakeholders, consolidate their perspectives on clinical AI implementation, and characterize the evidence gaps that future qualitative research should target.
Methods: Ovid-MEDLINE, EBSCO-CINAHL, ACM Digital Library, Science Citation Index-Web of Science, and Scopus were searched for primary qualitative studies on individuals' perspectives on any application of clinical AI worldwide (January 2014-April 2021). The definition of clinical AI includes both rule-based and machine learning-enabled or non-rule-based decision support tools. The language of the reports was not an exclusion criterion. Two independent reviewers performed title, abstract, and full-text screening with a third arbiter of disagreement. Two reviewers assigned the Joanna Briggs Institute 10-point checklist for qualitative research scores for each study. A single reviewer extracted free-text data relevant to clinical AI implementation, noting the stakeholders contributing to each excerpt. The best-fit framework synthesis used the Nonadoption, Abandonment, Scale-up, Spread, and Sustainability (NASSS) framework. To validate the data and improve accessibility, coauthors representing each emergent stakeholder group codeveloped summaries of the factors most relevant to their respective groups.
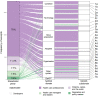
Results: The initial search yielded 4437 deduplicated articles, with 111 (2.5%) eligible for inclusion (median Joanna Briggs Institute 10-point checklist for qualitative research score, 8/10). Five distinct stakeholder groups emerged from the data: health care professionals (HCPs), patients, carers and other members of the public, developers, health care managers and leaders, and regulators or policy makers, contributing 1204 (70%), 196 (11.4%), 133 (7.7%), 129 (7.5%), and 59 (3.4%) of 1721 eligible excerpts, respectively. All stakeholder groups independently identified a breadth of implementation factors, with each producing data that were mapped between 17 and 24 of the 27 adapted Nonadoption, Abandonment, Scale-up, Spread, and Sustainability subdomains. Most of the factors that stakeholders found influential in the implementation of rule-based clinical AI also applied to non-rule-based clinical AI, with the exception of intellectual property, regulation, and sociocultural attitudes.
Conclusions: Clinical AI implementation is influenced by many interdependent factors, which are in turn influenced by at least 5 distinct stakeholder groups. This implies that effective research and practice of clinical AI implementation should consider multiple stakeholder perspectives. The current underrepresentation of perspectives from stakeholders other than HCPs in the literature may limit the anticipation and management of the factors that influence successful clinical AI implementation. Future research should not only widen the representation of tools and contexts in qualitative research but also specifically investigate the perspectives of all stakeholder HCPs and emerging aspects of non-rule-based clinical AI implementation.
Trial registration: PROSPERO (International Prospective Register of Systematic Reviews) CRD42021256005; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=256005.
International registered report identifier (irrid): RR2-10.2196/33145.
Keywords: artificial intelligence; computerized decision support; implementation; qualitative evidence synthesis; qualitative research; systematic review.
©Henry David Jeffry Hogg, Mohaimen Al-Zubaidy, Technology Enhanced Macular Services Study Reference Group, James Talks, Alastair K Denniston, Christopher J Kelly, Johann Malawana, Chrysanthi Papoutsi, Marion Dawn Teare, Pearse A Keane, Fiona R Beyer, Gregory Maniatopoulos. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 10.01.2023.
Conflict of interest statement
Conflicts of Interest: CJK is an employee of Google and owns stocks as part of the standard compensation package. PAK has acted as a consultant for Google, DeepMind, Roche, Novartis, Apellis, and BitFount and is an equity owner in Big Picture Medical. He has received speaker fees from Heidelberg Engineering, Topcon, Allergan, and Bayer. All other authors declare no competing interests.
Figures

References
-
- Zhang J, Whebell S, Gallifant J, Budhdeo S, Mattie H, Lertvittayakumjorn P, del Pilar Arias Lopez M, Tiangco BJ, Gichoya JW, Ashrafian H, Celi LA, Teo JT. An interactive dashboard to track themes, development maturity, and global equity in clinical artificial intelligence research. Lancet Digital Health. 2022 Apr;4(4):e212–3. doi: 10.1016/s2589-7500(22)00032-2. - DOI - PMC - PubMed
-
- Generating Evidence for Artificial Intelligence Based Medical Devices: A Framework for Training Validation and Evaluation. Geneva: World Health Organization; 2021.
-
- Yin J, Ngiam KY, Teo HH. Role of artificial intelligence applications in real-life clinical practice: systematic review. J Med Internet Res. 2021 Apr 22;23(4):e25759. doi: 10.2196/25759. https://www.jmir.org/2021/4/e25759/ v23i4e25759 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

