Glucocorticoids for croup in children
- PMID: 36626194
- PMCID: PMC9831289
- DOI: 10.1002/14651858.CD001955.pub5
Glucocorticoids for croup in children
Abstract
Background: Glucocorticoids are the mainstay for the treatment of croup. The existing evidence demonstrates that glucocorticoids are effective in the treatment of croup in children. However, updating the evidence on their clinical relevance in croup is imperative. This is an update to a review first published in 1999, and updated in 2004, 2011, and 2018.
Objectives: To investigate the effects and safety of glucocorticoids in the treatment of croup in children aged 18 years and below.
Search methods: We searched the Cochrane Library, which includes the Cochrane Central Register of Controlled Trials (CENTRAL; 2022 Issue 9), Ovid MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Ovid MEDLINE (1946 to 4 March 2022), Embase (Ovid) (1974 to 4 March 2022). We also searched the WHO ICTRP and ClinicalTrials.gov on 4 March 2022.
Selection criteria: We included randomised controlled trials (RCTs) in children (aged 18 years and below) with croup. We assessed the effect of glucocorticoids compared to the following: placebo, any other pharmacologic agents, any other glucocorticoids, any combination of other glucocorticoids, given by different modes of administration, or given in different doses. The included studies must have assessed at least one of our primary outcomes (defined as the change in croup score or return visits, (re)admissions to the hospital or both) or secondary outcomes (defined as the length of stay in hospital or emergency departments, patient improvement, use of additional treatments, or adverse events).
Data collection and analysis: Review authors independently extracted data, with another review author verified. We entered the data into Review Manager 5 for meta-analysis. Two review authors independently assessed studies for risk of bias using the Cochrane risk of bias tool. Two review authors assessed the certainty of the evidence for the primary outcomes using the GRADE approach.
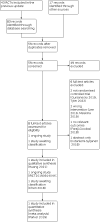
Main results: This updated review includes 45 RCTs with a total of 5888 children, an increase of two RCTs with 1323 children since the last update. We also identified one ongoing study and one study awaiting classification. We assessed most studies (98%) as at high or unclear risk of bias. Any glucocorticoid compared to placebo Compared to placebo, glucocorticoids may result in greater reductions in croup score after two hours (standardised mean difference (SMD) -0.65, 95% confidence interval (CI) -1.13 to -0.18; 7 RCTs, 426 children; low-certainty evidence); six hours (SMD -0.76, 95% CI -1.12 to -0.40; 11 RCTs, 959 children; low-certainty evidence); and 12 hours (SMD -1.03, 95% CI -1.53 to -0.53; 8 RCTs, 571 children; low-certainty evidence). The evidence for change in croup score after 24 hours is very uncertain (SMD -0.86, 95% CI -1.40 to -0.31; 8 RCTs, 351 children; very low-certainty evidence). One glucocorticoid compared to another glucocorticoid There was little to no difference between prednisolone and dexamethasone for reduction in croup score at two-hour post-baseline score (SMD 0.06, 95% CI -0.06 to 0.18; 1 RCT, 1231 children; high-certainty evidence). There was likely little to no difference between prednisolone and dexamethasone for reduction in croup score at six-hour post-baseline score (SMD 0.21, 95% CI -0.21 to 0.62; 1 RCT, 99 children; moderate-certainty evidence). However, dexamethasone probably reduced the return visits or (re)admissions for croup by almost half (risk ratio (RR) 0.55, 95% CI 0.28 to 1.11; 4 RCTs, 1537 children; moderate-certainty evidence), and showed a 28% reduction in the use of supplemental glucocorticoids as an additional treatment (RR 0.72, 95% CI 0.53 to 0.97; 2 RCTs, 926 children). Dexamethasone given in different doses Compared to 0.15 mg/kg, 0.60 mg/kg dexamethasone probably reduced the severity of croup as assessed by the croup scoring scale at 24-hour postbaseline score (SMD 0.63, 95% CI 0.16 to 1.10; 1 RCT, 72 children; moderate-certainty evidence); however, this was not the case at two hours (SMD -0.27, 95% CI -0.76 to 0.22; 2 RCTs, 861 children; high-certainty evidence). There was probably no reduction at six hours (SMD -0.45, 95% CI -1.26 to 0.35; 3 RCTs, 178 children; moderate-certainty evidence), and the evidence at 12 hours is very uncertain (SMD -0.60, 95% CI -4.39 to 3.19; 2 RCTs, 113 children; very low-certainty evidence). There was little to no difference between doses of dexamethasone in return visits or (re)admissions of children or both (RR 0.91, 95% CI 0.71 to 1.17; 3 RCTs, 949 children; high-certainty evidence) or length of stay in the hospital or emergency department (mean difference 0.12, 95% CI -0.32 to 0.56; 2 RCTs, 892 children). The need for additional treatments, such as epinephrine (RR 0.78, 95% CI 0.34 to 1.75; 2 RCTs, 885 children); intubation (risk difference 0.00, 95% CI -0.00 to 0.00; 2 RCTs, 861 children); or use of supplemental glucocorticoids (RR 0.77, 95% CI 0.51 to 1.15; 2 RCTs, 617 children), also did not differ between doses of dexamethasone. There were moderate to high levels of heterogeneity in the analyses for most comparisons. Adverse events were observed for some of the comparisons reported in the review.
Authors' conclusions: The evidence that glucocorticoids reduce symptoms of croup at two hours, shorten hospital stays, and reduce the rate of return visits or (re)admissions has not changed in this update. A smaller dose of 0.15 mg/kg of dexamethasone may be as effective as the standard dose of 0.60 mg/kg. More RCTs are needed to strengthen the evidence for effectiveness of low-dose dexamethasone at 0.15 mg/kg to treat croup.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Alex Aregbesola: declared that they have no conflict of interest. Clara Tam: declared that they have no conflict of interest. Asha Kothari: declared that they have no conflict of interest. Mê‐Linh Lê: declared that they have no conflict of interest. Mirna Ragheb: declared that they have no conflict of interest. Terry P Klassen: is an author of four of the included studies (Bjornson 2004; Klassen 1994; Klassen 1996; Klassen 1998).
Figures



































































































Update of
-
Glucocorticoids for croup in children.Cochrane Database Syst Rev. 2018 Aug 22;8(8):CD001955. doi: 10.1002/14651858.CD001955.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Jan 10;1:CD001955. doi: 10.1002/14651858.CD001955.pub5. PMID: 30133690 Free PMC article. Updated.
References
References to studies included in this review
Alshehr 2005 {published data only}
-
- Alshehr M, Almegamsi T, Hammdi A. Efficacy of a small dose of oral dexamethasone in croup. Biomedical Research 2005;65(1):65-72.
Amir 2006 {published data only}
-
- Amir L, Hubermann H, Halevi A, Mor M, Mimouni M, Waisman Y. Oral betamethasone versus intramuscular dexamethasone for the treatment of mild to moderate viral croup. Pediatric Emergency Care 2006;22(8):541-4. - PubMed
Bjornson 2004 {published and unpublished data}
-
- Bjornson C, Klassen TP, Williamson J, Brant R, Plint A, Bulloch B, et al. A randomized trial of single dose of oral dexamethasone for mild croup. New England Journal of Medicine 2004;351(13):1306-13. - PubMed
Cetinkaya 2004 {published data only}
-
- Cetinkaya F, Tufekci BS, Kutluk G. A comparison of nebulized budesonide, and intramuscular, and oral dexamethasone for treatment of croup. International Journal of Pediatric Otorhinolaryngology 2004;68(4):453-6. - PubMed
Chub‐Uppakarn 2007 {published data only}
-
- Chub-Uppakarn S, Sangsupawanich P. A randomized comparison of dexamethasone 0.15 mg/kg versus 0.6 mg/kg for the treatment of moderate to severe croup. International Journal of Pediatric Otorhinolaryngology 2007;71(3):473-7. - PubMed
Cruz 1995 {published data only}
-
- Cruz MN, Stewart G, Rosenberg N. Use of dexamethasone in the outpatient management of acute laryngotracheitis. Pediatrics 1995;96(2 Pt 1):220-3. - PubMed
Dobrovoljac 2012 {published data only}
-
- Dobrovoljac M, Geelhoed G. How fast does oral dexamethasone work in mild to moderately severe croup? A randomized double-blinded clinical trial. Emergency Medicine Australasia 2012;24(1):79-85. - PubMed
Donaldson 2003 {published data only}
-
- Donaldson D, Poleski D, Knipple E, Filips K, Reetz L, Pascula RG, et al. Intramuscular versus oral dexamethasone for the treatment of moderate-to-severe croup: a randomized double-blind trial. Academy of Emergency Medicine 2003;10(1):16-21. - PubMed
Duman 2005 {published data only}
-
- Duman M, Ozdemir D, Atasever S. Nebulized L-epinephrine and steroid combination in the treatment of moderate to severe croup. Clinical Drug Investigation 2005;25(3):183-9. - PubMed
Eboriadou 2010 {published data only}
-
- Eboriadou M, Chryssanthopoulou D, Stamoulis P, Damianidou L, Haidopoulou K. The effectiveness of local corticosteroids therapy in the management of mild to moderate viral croup. Minerva Pediatrica 2010;62(1):23-8. - PubMed
Eden 1964 {published data only}
-
- Eden A, Larkin VP. Corticosteroid treatment of croup. Pediatrics 1964;33:768-9. - PubMed
Eden 1967 {published data only}
-
- Eden AN, Kaufman A, Yu R. Corticosteroids and croup. Controlled double-blind study. JAMA 1967;200(5):403-4. - PubMed
Fifoot 2007 {published data only}
-
- Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup: a randomized, double-blinded clinical trial. Emergency Medicine Australasia 2007;19(1):51-8. - PubMed
Fitzgerald 1996 {published data only}
-
- Fitzgerald D, Mellis C, Johnson M, Allen H, Cooper P, Van Asperen P. Nebulized budesonide is as effective as nebulized adrenaline in moderately severe croup. Pediatrics 1996;97(5):722-5. - PubMed
Garbutt 2013 {published data only}
Geelhoed 1995a {published data only}
-
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362-8. - PubMed
Geelhoed 1995b {published data only}
-
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362-8. - PubMed
Geelhoed 1995c {published data only}
-
- Geelhoed GC, Macdonald WB. Oral and inhaled steroids in croup: a randomized, placebo‐controlled trial. Pediatric Pulmonology 1995;20(6):355-61. - PubMed
Geelhoed 1996a {published data only}
Geelhoed 2005 {published data only}
-
- Geelhoed GC. Budesonide offers no advantage when added to oral dexamethasone in the treatment of croup. Pediatric Emergency Care 2005;21(6):359-62. - PubMed
Godden 1997 {published data only}
Huang 2021 {published data only}
-
- Huang T, Xia Z, Li W. Efficacy of inhaled budesonide on serum inflammatory factors and quality of life among children with acute infectious laryngitis. American Journal of Otolaryngology - Head and Neck Medicine and Surgery 2021;42(1):102820. - PubMed
Husby 1993 {published data only}
-
- Mortensen S, Agertoft L, Husby S, Pedersen S. Pseudocroup treated with inhaled steroid (budesonide). A double-blind placebo-controlled trial. Ugeskrift for Laeger 1994;156(45):6661-3. - PubMed
James 1969 {published data only}
-
- James JA. Dexamethasone in croup. A controlled study. American Journal of Diseases of Children 1969;117(5):511-6. - PubMed
Johnson 1996 {published data only}
-
- Johnson DW, Schuh S, Koren G, Jaffee DM. Outpatient treatment of croup with nebulized dexamethasone. Archives of Pediatrics & Adolescent Medicine 1996;150(4):349-55. - PubMed
Johnson 1998 {published data only}
-
- Johnson DW, Jacobson S, Edney PC, Hadfield P, Mundy ME, Schuh S. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. New England Journal of Medicine 1998;339(8):498-503. - PubMed
Klassen 1994 {published data only}
-
- Klassen TP, Feldman ME, Watters LK, Sutcliffe T, Rowe PC. Nebulized budesonide for children with mild-to-moderate croup. New England Journal of Medicine 1994;331(5):285-9. - PubMed
Klassen 1996 {published data only}
-
- Klassen TP, Watters LK, Feldman ME, Sutcliffe T, Rowe PC. The efficacy of nebulized budesonide in dexamethasone‐treated outpatients with croup. Pediatrics 1996;97(4):463-6. - PubMed
Klassen 1998 {published data only}
-
- Klassen TP, Craig WR, Moher D, Osmond MH, Pasterkamp H, Sutcliffe T, et al. Nebulized budesonide and oral dexamethasone for treatment of croup: a randomized controlled trial. JAMA 1998;279(20):1629-32. - PubMed
Koren 1983 {published data only}
-
- Koren GF. Corticosteroid treatment of laryngotracheitis v spasmodic croup in children. American Journal of Diseases of Children 1983;137(10):941-4. - PubMed
Kuusela 1988 {published data only}
-
- Kuusela AL, Vesikari T. A randomized double-blind, placebo-controlled trial of dexamethasone and racemic epinephrine in the treatment of croup. Acta Paediatrica Scandinavica 1988;77(1):99-104. - PubMed
Leipzig 1979 {published data only}
-
- Leipzig B, Oski FA, Cummings CW, Stockman JA, Swender P. A prospective randomized study to determine the efficacy of steroids in treatment of croup. Journal of Pediatrics 1979;94(2):194-6. - PubMed
Luria 2001 {published data only}
-
- Luria JW, Gonzalez-del-Rey JA, DiGuilio GA, McAneney CM, Olsen JJ, Ruddy RM. Effectiveness of oral or nebulized dexamethasone for children with mild croup. Archives of Pediatrics & Adolescent Medicine 2001;155(12):1340-5. - PubMed
Martinez Fernandez 1993 {published data only}
-
- Martinez Fernandez A, Sanchez GE, Rica EI, Echaniz UI, Alonso DM, Vilella CM, et al. Randomized double-blind study of treatment of croup with adrenaline and/or dexamethasone in children. Anales Españoles de Pediatria 1993;38:29-32. - PubMed
Massicotte 1973 {published data only}
-
- Massicotte P, Tetreault L. Evaluation of methyl‐prednisolone in the treatment of acute laryngitis in children. Unión Médicale du Canada 1973;102(10):2064-72. - PubMed
Parker 2019 {published data only}
-
- Parker CM, Cooper MN. Prednisolone versus dexamethasone for croup: a randomized controlled trial. Pediatrics 2019;144(3):e20183772. - PubMed
Rittichier 2000 {published data only}
-
- Rittichier KK, Ledwith CA. Outpatient treatment of moderate croup with dexamethasone: intramuscular versus oral dosing. Pediatrics 2000;106(6):1344-8. - PubMed
Roberts 1999 {published data only}
-
- Roberts GW, Master VV, Staugas RE, Raftos JV, Parsons DW, Coulthard KP, et al. Repeated dose inhaled budesonide versus placebo in the treatment of croup. Journal of Paediatrics and Child Health 1999;35(2):170-4. - PubMed
Roorda 1998 {published data only}
-
- Roorda RJ, Walhof CM. Effects of inhaled fluticasone propionate administered with metered dose inhaler and spacer in mild to moderate croup: a negative preliminary report. Pediatric Pulmonology 1998;25(2):114-7. - PubMed
Skowron 1966a {published data only}
Skowron 1966a and b {published data only}
Skowron 1966b {published data only}
Soleimani 2013 {published data only}
-
- Soleimani G, Daryadel A, Moghadam AA, Sharif MR. The comparison of oral and IM dexamethasone efficacy in croup treatment. Journal of Comprehensive Pediatrics 2013;4(4):175-8.
Sparrow 2006 {published data only}
Super 1989 {published data only}
-
- Super DM, Cartelli NA, Brooks LJ, Lembo RM, Kumar ML. A prospective randomized double‐blind study to evaluate the effect of dexamethasone in acute laryngotracheitis. Journal of Pediatrics 1989;115(2):323-9. - PubMed
Tibballs 1992 {published data only}
-
- Tibballs J, Shann FA, Landau LI. Placebo-controlled trial of prednisolone in children intubated for croup. Lancet 1992;340(8822):745-8. - PubMed
Vad Pedersen 1998 {published data only}
-
- Vad Pedersen L, Dahl M, Falk-Petersen HE, Larsen SE. Inhaled budesonide versus intramuscular dexamethasone in the treatment of pseudocroup [Inhaleret budesonid versus dexamethasone i.m. til behandling at pseudocroup]. Ugeskrift for Laeger 1998;160(15):2253-6. - PubMed
Von Mühlendahl 1982 {published data only}
-
- Von Mühlendahl KE, Kahn D, Spohr HL, Dressler F. Steroid treatment of pseudo‐croup. Helvetica Paediatrica Acta 1982;37(5):431-6. - PubMed
References to studies excluded from this review
Anene 1996 {published data only}
-
- Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double‐blind, placebo‐controlled trial. Critical Care Medicine 1996;24(10):1666-9. - PubMed
Bollobas 1965 {published data only}
-
- Bollobas B. On local adrenocortical hormone treatment of rhinolaryngologic diseases. Zeitschrift für Laryngologie, Rhinologie, Otologie und Ihre Grenzgebiete 1965;44(7):476-81. - PubMed
Cichy 1983 {published data only}
-
- Cichy M, Pawlik J. Treatment of subglottic laryngitis in children. Otolaryngologia Polska 1983;37(1):11-3. - PubMed
Connolly 1969 {published data only}
-
- Connolly JH, Field C, Glasgow J. A double blind trial of prednisolone in epidemic bronchiolitis due to respiratory syncytial virus. Acta Pediatrica Scandinavica 1969;58(2):116-20. - PubMed
Couser 1992 {published data only}
-
- Couser RJ, Ferrara TB, Falde B, Johnson K, Schilling CG, Hoekstra RE. Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. Journal of Pediatrics 1992;121(4):591-6. - PubMed
Eghbali 2016 {published data only}
Faghihinia 2007 {published data only}
-
- Faghihinia J. A comparison between intramuscular dexamethasone and fluticasone propionate inhaler in treatment of croup. World Allergy Organization Journal 2007;S104:327.
Faraji‐Goodarzi 2018 {published data only}
-
- Faraji-Goodarzi M, Taee N, Mohammadi-Kamalvand M. Comparison of the effect of cold drink and dexamethasone, and their combined effect on children with croup. Drug Research 2018;68(4):185-8. - PubMed
Flisberg 1973 {published data only}
-
- Flisberg K, Olsholt R. Pseudocroup with stridor. Acta Oto-Laryngologica 1973;76(4):295-9. - PubMed
Freezer 1990 {published data only}
-
- Freezer N, Butt W, Phelan P. Steroids in croup: do they increase the incidence of successful extubation? Anaesthesia and Intensive Care 1990;18(2):224-8. - PubMed
Gill 2017 {published data only}
Goddard 1967 {published data only}
-
- Goddard JE, Phillips OC, Marcy JH. Betamethasone for prophylaxis of postintubation inflammation: a double‐blind study. Anesthesia and Analgesia 1967;46(3):348-53. - PubMed
Gursanscky 2019 {published data only}
-
- Gursanscky L. Prednisolone versus dexamethasone for croup. Journal of Paediatrics and Child Health 2019;55(12):1511.
Haque 1981 {published data only}
-
- Haque KN. Efficacy of dexamethasone in acute laryngotracheobronchitis (croup). Saudi Medical Journal 1981;2(3):143-5.
Havaldar 1997 {published data only}
-
- Havaldar PV. Dexamethasone in laryngeal diphtheritic croup. Annals of Tropical Paediatrics 1997;17(1):21-3. - PubMed
Kelley 1992 {published data only}
-
- Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. American Journal of Emergency Medicine 1992;10(3):181-3. - PubMed
Kotaniemi‐Syrjanen 2018 {published data only}
-
- Kotaniemi-Syrjanen A, Klemola T, Koponen P, Aito H, Malmstrom K, Malmberg P, et al. Intermittent tiotropium in early childhood wheezing? Preliminary safety results of a pilot study. European Respiratory Journal 2018;52(Suppl 62):PA1042. [DOI: 10.1183/13993003.congress-2018.PA1042] - DOI
Kunkel 1996 {published data only}
-
- Kunkel NC, Baker MD. Use of racemic epinephrine, dexamethasone, and mist in the outpatient management of croup. Pediatric Emergency Care 1996;12(3):156-9. - PubMed
Ledwith 1995 {published data only}
-
- Ledwith CA, Shea LM, Mauro RD. Safety and efficacy of nebulized racemic epinephrine in conjunction with oral dexamethasone and mist in the outpatient treatment of croup. Annals of Emergency Medicine 1995;25(3):331-7. - PubMed
Lee 2019 {published data only}
-
- Lee JH, Jung JY, Lee HJ, Kim DK, Kwak YH, Chang I, et al. Efficacy of low-dose nebulized epinephrine as treatment for croup: a randomized, placebo-controlled, double-blind trial. American Journal of Emergency Medicine 2019;31(12):2171-6. - PubMed
Martensson 1960 {published data only}
-
- Martensson B, Nilsson G, Torbjar J. The effect of corticosteroids in the treatment of pseudo‐croup. Acta Oto-Laryngologica 1960;158(Suppl):62-71. - PubMed
McDonogh 1994 {published data only}
-
- McDonogh AJ. The use of steroids and nebulised adrenaline in the treatment of viral croup over a seven year period at a district hospital. Anaesthesia and Intensive Care 1994;22(2):175-8. - PubMed
Meskina 2019 {published data only}
-
- Meskina ER, Khadisova MK, Tselipanova EE. Efficacy of a homeopathic drug in combination treatment of acute obstructive laryngitis in children. Voprosy Prakticheskoi Pediatrii 2019;14(4):36-43.
Mohammadzadeh 2014 {published data only}
-
- Mohammadzadeh I, Noorouzi AR, Nakhjavani N, Barari-Savadkoohi R, Mohammadpor-Mir A, Alizadeh-Navaei R. The effect of dexamethasone and nebulised L-epinephrine in treatment of croup. Journal of Babol University of Medical Sciences 2014;16(2):12-6.
NCT01748162 {published data only}
-
- NCT01748162. Management of recurrent croup. clinicaltrials.gov/ct2/show/NCT01748162 (first received 12 December 2012).
Novik 1960 {published data only}
-
- Novik A. Corticosteroid treatment of non-diphtheric croup. Acta Oto-Laryngologica 1960;158(Suppl):20-2. - PubMed
Osváth 1994 {published data only}
-
- Osváth P, Kelenhegyi K, Szánthó A. Management of childhood pseudocroup with budesonide inhalation. Orvosi Hetilap 1994;135(46):2535-7. - PubMed
Prendergast 1994 {published data only}
-
- Prendergast M, Jones JS, Hartman D. Racemic epinephrine in the treatment of laryngotracheitis: can we identify children for outpatient therapy? American Journal of Emergency Medicine 1994;12(6):613-6. - PubMed
Rizos 1998 {published data only}
-
- Rizos J, DiGravio B, Sehl M, Tallon J. The disposition of children with croup treated with racemic epinephrine and dexamethasone in the emergency department. Journal of Emergency Medicine 1998;16(4):535-9. - PubMed
Roked 2015 {published data only}
-
- Roked F, Atkinson M, Hartshorn S. Best practice: one or two doses of dexamethasone for the treatment of croup? Archives of Disease in Childhood 2015;100(Suppl 3):A40-1.
Ross 1969 {published data only}
-
- Ross JA. Special problems in acute laryngotracheobronchitis. Laryngoscope 1969;79(7):1218-26. - PubMed
Serra 1997 {published data only}
-
- Serra A, Bonarrigo A, Cupido GF, Manciagli M, Pantalena V, Raso D, et al. Experience with flunisolide in inflammatory diseases in otorhinolaryngology. Multicentric trial [Impiego di flunisolide nel trattamento di laringiti e sinusiti]. Otorinolaringologia 1997;47(3):137-44.
Sumboonnanonda 1997 {published data only}
-
- Sumboonnanonda A, Suwanjutha S, Sirinavin S. Randomized controlled trial of dexamethasone in infectious croup. Journal of the Medical Association of Thailand 1997;80(4):262-5. - PubMed
Sussman 1964 {published data only}
-
- Sussman S, Grossman M, Magoffin R, Schieble J. Dexamethasone (16 alpha‐methyl, 9 alpha fluoroprednisolone) in obstructive respiratory tract infections in children. Pediatrics 1964;34:851-5. - PubMed
Tal 1983 {published data only}
-
- Tal A, Bavilski C, Yohai D. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71(1):13-8. - PubMed
Tellez 1991 {published data only}
-
- Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW. Dexamethasone in the prevention of postextubation stridor in children. Journal of Pediatrics 1991;118(2):289-94. - PubMed
Tyler 2022 {published data only}
Wilhelmi 1976 {published data only}
-
- Wilhelmi J. High dosage rectal prednisone therapy (Rectodelt 100) in viral croup of the small child. Medizinische Monatsschrift 1976;30(10):467-9. - PubMed
References to studies awaiting assessment
Chen 2018 {published data only}
-
- Chen QP, Zhou RF, Zhang YM, Yang L. Efficacy of systemic glucocorticoids combined with inhaled steroid on children with acute laryngitis. Zhonghua er bi yan hou tou jing wai ke za zhi [Chinese Journal of Otorhinolaryngology Head and Neck Surgery] 2018;53(1):53-6. - PubMed
References to ongoing studies
IRCT20190914044765N1 {published data only}
-
- IRCT20190914044765N1. Comparison the effect of oral and intravenous dexamethasone effect on the mild and moderate croup treatment in children. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190914044765N1 (first received 24 September 2019).
Additional references
Abrams 2005
-
- Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823-44. - PubMed
Alberta Medical Association 2008
-
- Alberta Medical Association Toward Optimized Practice Working Group for Croup. Guideline for the diagnosis and management of croup. actt.albertadoctors.org/CPGs/Lists/CPGDocumentList/croup-guideline.pdf (accessed prior to 22 December 2022).
Atkins 2004
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-101. - PubMed
Bjornson 2013
Bjornson 2016
-
- Bjornson C, Williamson J, Johnson D. Telephone Out Patient Score: the derivation and validation of a telephone follow-up assessment tool for use in clinical research in children with croup. Pediatric Emergency Care 2016;32(5):290-7. - PubMed
Brown 2002
-
- Brown JC. The management of croup. British Medical Bulletin 2002;61:189-202. - PubMed
Chan 2001
Cherry 1979
-
- Cherry JD. The treatment of croup: continued controversy due to failure of recognition of historic, ecologic, etiologic and clinical perspectives. Journal of Pediatrics 1979;94(2):352-4. - PubMed
Cherry 2008
-
- Cherry JD. Clinical practice. Croup. New England Journal of Medicine 2008;358(4):384-91. - PubMed
Denny 1983
-
- Denny FW, Murphy TF, Clyde WA Jr, Collier AM, Henderson FW. Croup: an 11-year study in a pediatric practice. Pediatrics 1983;71(6):871-6. - PubMed
Dobrovoljac 2009
-
- Dobrovoljac M, Geelhoed GC. 27 years of croup: an update highlighting the effectiveness of 0.15 mg/kg of dexamethasone. Emergency Medicine Australasia 2009;21(4):309-14. - PubMed
Downes 1975
-
- Downes JJ, Raphaelly RC. Pediatric intensive care. Anesthesiology 1975;43:238-50. - PubMed
Egger 1997
Follmann 1992
-
- Follmann D, Elliott P, Suh l, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(2):769-73. - PubMed
Furukawa 2006
Geelhoed 1995
-
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362-8. - PubMed
Geelhoed 1996b
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 23 February 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Griffin 2000
Hartling 2014
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2011b
Jüni 2001
Kairys 1989
-
- Kairys SW, Olmstead EM, O'Connor GT. Steroid treatment of laryngotracheitis: a meta-analysis of the evidence from randomized trials. Pediatrics 1989;83(5):683-93. - PubMed
Light 1984
-
- Light RS, Pillemar DB. Summing Up: The Science of Reviewing Research. Cambridge: Harvard University Press, 1984.
Microsoft Excel [Computer program]
-
- Microsoft Excel. Redmond (WA): Microsoft Corporation, 2016.
Ouzzani 2016
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rosychuk 2010
Sizar 2021
-
- Sizar O, Carr B. Croup. www.ncbi.nlm.nih.gov/books/NBK431070/?report=classic (accessed prior to 23 November 2022).
Van Bever 1999
-
- Van Bever HP, Wieringa MH, Weyler JJ, Nelen VJ, Fortuin M, Vermeire PA. Croup and recurrent croup: their association with asthma and allergy. An epidemiological study on 5-8-year-old children. European Journal of Pediatrics 1999;158(3):253-7. - PubMed
Weinberg 2009
-
- Weinberg GA, Hall CB, Iwane MK, Poehling KA, Edwards KM, Griffin MR, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. Journal of Pediatrics 2009;154(5):694-9. - PubMed
Westley 1978
-
- Westley CR, Cotton EK, Brooks JG. Nebulized racemicepinephrine by IPPB for the treatment of croup. American Journal of Diseases in Children 1978;132(5):484-7. - PubMed
References to other published versions of this review
Ausejo 1999
Ausejo 2000
Gates 2018
Gates 2019
Russell 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

