Low nephron endowment increases susceptibility to renal stress and chronic kidney disease
- PMID: 36626229
- PMCID: PMC9977438
- DOI: 10.1172/jci.insight.161316
Low nephron endowment increases susceptibility to renal stress and chronic kidney disease
Abstract
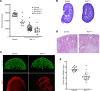
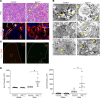
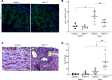
Preterm birth results in low nephron endowment and increased risk of acute kidney injury (AKI) and chronic kidney disease (CKD). To understand the pathogenesis of AKI and CKD in preterm humans, we generated potentially novel mouse models with a 30%-70% reduction in nephron number by inhibiting or deleting Ret tyrosine kinase in the developing ureteric bud. These mice developed glomerular and tubular hypertrophy, followed by the transition to CKD, recapitulating the renal pathological changes seen in humans born preterm. We injected neonatal mice with gentamicin, a ubiquitous nephrotoxic exposure in preterm infants, and detected more severe proximal tubular injury in mice with low nephron number compared with controls with normal nephron number. Mice with low nephron number had reduced proliferative repair with more rapid development of CKD. Furthermore, mice had more profound inflammation with highly elevated levels of MCP-1 and CXCL10, produced in part by damaged proximal tubules. Our study directly links low nephron endowment with postnatal renal hypertrophy, which in this model is maladaptive and results in CKD. Underdeveloped kidneys are more susceptible to gentamicin-induced AKI, suggesting that AKI in the setting of low nephron number is more severe and further increases the risk of CKD in this vulnerable population.
Keywords: Chronic kidney disease; Nephrology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

