Proline and glucose metabolic reprogramming supports vascular endothelial and medial biomass in pulmonary arterial hypertension
- PMID: 36626231
- PMCID: PMC9977503
- DOI: 10.1172/jci.insight.163932
Proline and glucose metabolic reprogramming supports vascular endothelial and medial biomass in pulmonary arterial hypertension
Abstract
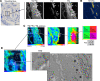
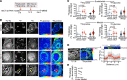
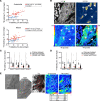
In pulmonary arterial hypertension (PAH), inflammation promotes a fibroproliferative pulmonary vasculopathy. Reductionist studies emphasizing single biochemical reactions suggest a shift toward glycolytic metabolism in PAH; however, key questions remain regarding the metabolic profile of specific cell types within PAH vascular lesions in vivo. We used RNA-Seq to profile the transcriptome of pulmonary artery endothelial cells (PAECs) freshly isolated from an inflammatory vascular injury model of PAH ex vivo, and these data were integrated with information from human gene ontology pathways. Network medicine was then used to map all aa and glucose pathways to the consolidated human interactome, which includes data on 233,957 physical protein-protein interactions. Glucose and proline pathways were significantly close to the human PAH disease module, suggesting that these pathways are functionally relevant to PAH pathobiology. To test this observation in vivo, we used multi-isotope imaging mass spectrometry to map and quantify utilization of glucose and proline in the PAH pulmonary vasculature at subcellular resolution. Our findings suggest that elevated glucose and proline avidity underlie increased biomass in PAECs and the media of fibrosed PAH pulmonary arterioles. Overall, these data show that anabolic utilization of glucose and proline are fundamental to the vascular pathology of PAH.
Keywords: Amino acid metabolism; Cardiology; Endothelial cells; Fibrosis; Pulmonology.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

