Increased expression of GPX4 promotes the tumorigenesis of thyroid cancer by inhibiting ferroptosis and predicts poor clinical outcomes
- PMID: 36626251
- PMCID: PMC9876627
- DOI: 10.18632/aging.204473
Increased expression of GPX4 promotes the tumorigenesis of thyroid cancer by inhibiting ferroptosis and predicts poor clinical outcomes
Abstract
Background: Ferroptosis plays a critical role in suppressing cancer progression, and its essential regulator is glutathione peroxidase 4 (GPX4). High GPX4 expression can inhibit accumulation of iron, thus suppressing ferroptosis. However, its function in thyroid cancer has not been fully illuminated. Here, we explore the effect of GPX4 on thyroid cancer tumorigenesis and prognosis.
Methods: Based on The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases, GPX4 expression was investigated in cancer tissues and adjacent tissues. We determined the biological functions of GPX4-associated differentially expressed genes (DEGs) by using the "clusterProfiler" R package. In addition, the predictive value of GPX4 in thyroid cancer was assessed by using Cox regression analysis and nomograms. Finally, we conducted several in vitro experiments to determine the influence of GPX4 expression on proliferation and ferroptosis in thyroid cancer cells.
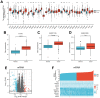
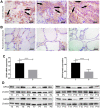
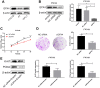
Results: GPX4 expression was obviously elevated in thyroid cancer tissues compared with normal tissues. Biological function analysis indicated enrichment in muscle contraction, contractile fiber, metal ion transmembrane transporter activity, and complement and coagulation cascades. GPX4 overexpression was associated with stage T3-T4 and pathologic stage III-IV in thyroid cancer patients. Cox regression analysis indicated that GPX4 may be a risk factor for the overall survival of thyroid cancer patients. In vitro research showed that knockdown of GPX4 suppressed proliferation and induced ferroptosis in thyroid cancer cells.
Conclusions: GPX4 overexpression in thyroid cancer might play an essential role in tumorigenesis and may have prognostic value for thyroid cancer patients.
Keywords: GPX4; clinicopathological features; ferroptosis; prognostic biomarker; thyroid cancer.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

