Metabolic reprogramming and its clinical implication for liver cancer
- PMID: 36626639
- PMCID: PMC10315435
- DOI: 10.1097/HEP.0000000000000005
Metabolic reprogramming and its clinical implication for liver cancer
Abstract
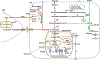
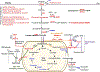
Cancer cells often encounter hypoxic and hypo-nutrient conditions, which force them to make adaptive changes to meet their high demands for energy and various biomaterials for biomass synthesis. As a result, enhanced catabolism (breakdown of macromolecules for energy production) and anabolism (macromolecule synthesis from bio-precursors) are induced in cancer. This phenomenon is called "metabolic reprogramming," a cancer hallmark contributing to cancer development, metastasis, and drug resistance. HCC and cholangiocarcinoma (CCA) are 2 different liver cancers with high intertumoral heterogeneity in terms of etiologies, mutational landscapes, transcriptomes, and histological representations. In agreement, metabolism in HCC or CCA is remarkably heterogeneous, although changes in the glycolytic pathways and an increase in the generation of lactate (the Warburg effect) have been frequently detected in those tumors. For example, HCC tumors with activated β-catenin are addicted to fatty acid catabolism, whereas HCC tumors derived from fatty liver avoid using fatty acids. In this review, we describe common metabolic alterations in HCC and CCA as well as metabolic features unique for their subsets. We discuss metabolism of NAFLD as well, because NAFLD will likely become a leading etiology of liver cancer in the coming years due to the obesity epidemic in the Western world. Furthermore, we outline the clinical implication of liver cancer metabolism and highlight the computation and systems biology approaches, such as genome-wide metabolic models, as a valuable tool allowing us to identify therapeutic targets and develop personalized treatments for liver cancer patients.
Copyright © 2023 American Association for the Study of Liver Diseases.
Conflict of interest statement
CONFLICT OF INTEREST
Nothing to report.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

