Engineered Nonviral Protein Cages Modified for MR Imaging
- PMID: 36626688
- PMCID: PMC9945100
- DOI: 10.1021/acsabm.2c00892
Engineered Nonviral Protein Cages Modified for MR Imaging
Abstract
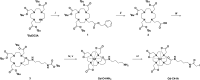
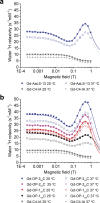
Diagnostic medical imaging utilizes magnetic resonance (MR) to provide anatomical, functional, and molecular information in a single scan. Nanoparticles are often labeled with Gd(III) complexes to amplify the MR signal of contrast agents (CAs) with large payloads and high proton relaxation efficiencies (relaxivity, r1). This study examined the MR performance of two structurally unique cages, AaLS-13 and OP, labeled with Gd(III). The cages have characteristics relevant for the development of theranostic platforms, including (i) well-defined structure, symmetry, and size; (ii) the amenability to extensive engineering; (iii) the adjustable loading of therapeutically relevant cargo molecules; (iv) high physical stability; and (v) facile manufacturing by microbial fermentation. The resulting conjugates showed significantly enhanced proton relaxivity (r1 = 11-18 mM-1 s-1 at 1.4 T) compared to the Gd(III) complex alone (r1 = 4 mM-1 s-1). Serum phantom images revealed 107% and 57% contrast enhancements for Gd(III)-labeled AaLS-13 and OP cages, respectively. Moreover, proton nuclear magnetic relaxation dispersion (1H NMRD) profiles showed maximum relaxivity values of 50 mM-1 s-1. Best-fit analyses of the 1H NMRD profiles attributed the high relaxivity of the Gd(III)-labeled cages to the slow molecular tumbling of the conjugates and restricted local motion of the conjugated Gd(III) complex.
Keywords: NMRD; gadolinium; magnetic resonance imaging; magnetism; nonviral protein cages.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

