Racial and Ethnic Disparities in Opioid Access and Urine Drug Screening Among Older Patients With Poor-Prognosis Cancer Near the End of Life
- PMID: 36626695
- PMCID: PMC10414726
- DOI: 10.1200/JCO.22.01413
Racial and Ethnic Disparities in Opioid Access and Urine Drug Screening Among Older Patients With Poor-Prognosis Cancer Near the End of Life
Abstract
Purpose: To characterize racial and ethnic disparities and trends in opioid access and urine drug screening (UDS) among patients dying of cancer, and to explore potential mechanisms.
Methods: Among 318,549 non-Hispanic White (White), Black, and Hispanic Medicare decedents older than 65 years with poor-prognosis cancers, we examined 2007-2019 trends in opioid prescription fills and potency (morphine milligram equivalents [MMEs] per day [MMEDs]) near the end of life (EOL), defined as 30 days before death or hospice enrollment. We estimated the effects of race and ethnicity on opioid access, controlling for demographic and clinical factors. Models were further adjusted for socioeconomic factors including dual-eligibility status, community-level deprivation, and rurality. We similarly explored disparities in UDS.
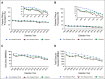
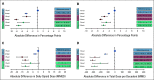
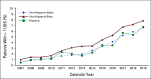
Results: Between 2007 and 2019, White, Black, and Hispanic decedents experienced steady declines in EOL opioid access and rapid expansion of UDS. Compared with White patients, Black and Hispanic patients were less likely to receive any opioid (Black, -4.3 percentage points, 95% CI, -4.8 to -3.6; Hispanic, -3.6 percentage points, 95% CI, -4.4 to -2.9) and long-acting opioids (Black, -3.1 percentage points, 95% CI, -3.6 to -2.8; Hispanic, -2.2 percentage points, 95% CI, -2.7 to -1.7). They also received lower daily doses (Black, -10.5 MMED, 95% CI, -12.8 to -8.2; Hispanic, -9.1 MMED, 95% CI, -12.1 to -6.1) and lower total doses (Black, -210 MMEs, 95% CI, -293 to -207; Hispanic, -179 MMEs, 95% CI, -217 to -142); Black patients were also more likely to undergo UDS (0.5 percentage points; 95% CI, 0.3 to 0.8). Disparities in EOL opioid access and UDS disproportionately affected Black men. Adjustment for socioeconomic factors did not attenuate the EOL opioid access disparities.
Conclusion: There are substantial and persistent racial and ethnic inequities in opioid access among older patients dying of cancer, which are not mediated by socioeconomic variables.
Conflict of interest statement
Racial and Ethnic Disparities in Opioid Access and Urine Drug Screening Among Older Patients With Poor-Prognosis Cancer Near the End of Life
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Andrea C. Enzinger
David M. Cutler
Cheryl R. Clark
Narjust Florez
Alexi A. Wright
No other potential conflicts of interest were reported.
Figures



Comment in
-
Examining Racial and Ethnic Inequities in Opioid Prescribing and Risk Screening Among Patients With Advanced Cancer.J Clin Oncol. 2023 May 10;41(14):2474-2477. doi: 10.1200/JCO.22.02879. Epub 2023 Feb 24. J Clin Oncol. 2023. PMID: 36827632 No abstract available.
References
-
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. : Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J Pain Symptom Manage 51:1070-1090.e9, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

