BAY 81-8973 Efficacy and Safety in Previously Untreated and Minimally Treated Children with Severe Hemophilia A: The LEOPOLD Kids Trial
- PMID: 36626898
- PMCID: PMC9831689
- DOI: 10.1055/s-0042-1757876
BAY 81-8973 Efficacy and Safety in Previously Untreated and Minimally Treated Children with Severe Hemophilia A: The LEOPOLD Kids Trial
Abstract
Introduction: BAY 81-8973, a full-length recombinant factor VIII for hemophilia A treatment, has been extensively evaluated in previously treated patients in the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials.
Aim: To assess BAY 81-8973 efficacy and safety when used for bleed prophylaxis and treatment in previously untreated/minimally treated patients (PUPs/MTPs).
Methods: In this phase III, multicenter, open-label, uncontrolled study, PUPs/MTPs (<6 years old) with severe hemophilia A received BAY 81-8973 (15-50 IU/kg) at least once weekly as prophylaxis. Primary efficacy endpoint was the annualized bleeding rate (ABR) within 48 hours after prophylaxis infusion. Adverse events and immunogenicity were assessed. Patients who developed inhibitors were offered immune tolerance induction (ITI) treatment in an optional extension phase.
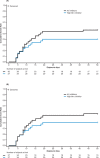
Results: Fifty-two patients were enrolled, with 43 patients (mean age: 13.6 months) treated. Median (interquartile range) ABR for all bleeds within 48 hours of prophylaxis infusion was 0.0 (0.0-1.8) among patients without inhibitors (n = 20) and 0.0 (0.0-2.2) among all patients. As expected, inhibitors were the most frequent treatment-related adverse event (high titer: 17 [39.5%] patients; low titer: 6 [13.9%] patients). Six of 12 patients who underwent ITI treatment in the extension phase (high titer [n = 5], low titer [n = 1]) achieved a negative inhibitor titer.
Conclusion: BAY 81-8973 was effective for bleed prevention and treatment in PUPs/MTPs. The observed inhibitor rate was strongly influenced by a cluster of inhibitor cases, and consequently, slightly higher than in other PUP/MTP studies. Overall, the BAY 81-8973 benefit-risk profile remains unchanged and supported by ongoing safety surveillance. Immune tolerance can be achieved with BAY 81-8973.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
R.L. has received consultancy fee from Bayer, SOBI, Novo Nordisk, Baxter/Shire, Pfizer, and Sanofi. A.K.C.C. has received grants from Octapharma, Novo Nordisk, Bayer, Sanofi, Sobi, BMS, I-ACT, and AceAge, honoraria from Bayer, Novo Nordisk, Takeda, Roche, and CSL, jointly holds patent # 20180177924 (Application # 15/735,306) “Medical Devices, Systems, and Methods Utilizing Antithrombin-Heparin Composition,” and advisory fees from Bayer, Daiichi Sankyo, and CSL Behring. M.E.M. has received consultant, advisor, and speaker fees from Bayer, CSL Behring, BioMarin, Grifols, Kedrion, LFB, Novo Nordisk, Pfizer, Roche, Sobi, Sanofi, Spark Therapeutics, Uniqure, Takeda, and Octopharma. G.K. has received grants from Alnylam, Bayer, BPL, Opko Biologics, Pfizer, Roche, and Takeda, and consulting fees and honoraria from Alnylam, Bayer, BioMarin Pharmaceutical, CSL, NovoNordisk, Opko Biologics, Pfizer, Takeda, Roche, Sanofi, and Uniquore. O.A. is an employee of Bayer US LLC. B.B. is an employee and stockholder of Bayer AG. D.T.-S. is an employee of Bayer Pharma AG. S.S.-T. and H.G. have no conflicts of interest to declare.
Figures

References
-
- Srivastava A, Santagostino E, Dougall A.WFH guidelines for the management of hemophilia, 3rd edition Haemophilia 202026(Suppl 6):1–158. - PubMed
-
- Valentino L A. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8(09):1895–1902. - PubMed
-
- Nilsson I M, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232(01):25–32. - PubMed
-
- The Orthopaedic Outcome Study Group . Aledort L M, Haschmeyer R H, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. J Intern Med. 1994;236(04):391–399. - PubMed
-
- Brackmann H H, Eickhoff H J, Oldenburg J, Hammerstein U. Long-term therapy and on-demand treatment of children and adolescents with severe haemophilia A: 12 years of experience. Haemostasis. 1992;22(05):251–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

