RNA splicing dysregulation and the hallmarks of cancer
- PMID: 36627445
- PMCID: PMC10132032
- DOI: 10.1038/s41568-022-00541-7
RNA splicing dysregulation and the hallmarks of cancer
Abstract
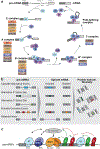
Dysregulated RNA splicing is a molecular feature that characterizes almost all tumour types. Cancer-associated splicing alterations arise from both recurrent mutations and altered expression of trans-acting factors governing splicing catalysis and regulation. Cancer-associated splicing dysregulation can promote tumorigenesis via diverse mechanisms, contributing to increased cell proliferation, decreased apoptosis, enhanced migration and metastatic potential, resistance to chemotherapy and evasion of immune surveillance. Recent studies have identified specific cancer-associated isoforms that play critical roles in cancer cell transformation and growth and demonstrated the therapeutic benefits of correcting or otherwise antagonizing such cancer-associated mRNA isoforms. Clinical-grade small molecules that modulate or inhibit RNA splicing have similarly been developed as promising anticancer therapeutics. Here, we review splicing alterations characteristic of cancer cell transcriptomes, dysregulated splicing's contributions to tumour initiation and progression, and existing and emerging approaches for targeting splicing for cancer therapy. Finally, we discuss the outstanding questions and challenges that must be addressed to translate these findings into the clinic.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
RKB is an inventor on patent applications filed by Fred Hutchinson Cancer Center related to modulating splicing for cancer therapy. OA is an inventor on a patent application filed by The Jackson Laboratory related to modulating splicing factors.
Figures




References
-
-
Wang ET et al. Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476, doi: 10.1038/nature07509 (2008).
Landmark study using RNA-seq to quantify isoform expression across tissues.
-
-
-
Kahles A et al. Comprehensive Analysis of Alternative Splicing Across Tumors from 8,705 Patients. Cancer Cell 34, 211–224.e216, doi: 10.1016/j.ccell.2018.07.001 (2018).
Landmark study identifying splicing alterations across tumor types.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

