Predictors of the experience of a Cytosponge test: analysis of patient survey data from the BEST3 trial
- PMID: 36627580
- PMCID: PMC9832657
- DOI: 10.1186/s12876-022-02630-1
Predictors of the experience of a Cytosponge test: analysis of patient survey data from the BEST3 trial
Abstract
Background: The Cytosponge is a cell-collection device, which, coupled with a test for trefoil factor 3 (TFF3), can be used to diagnose Barrett's oesophagus, a precursor condition to oesophageal adenocarcinoma. BEST3, a large pragmatic, randomised, controlled trial, investigated whether offering the Cytosponge-TFF3 test would increase detection of Barrett's. Overall, participants reported mostly positive experiences. This study reports the factors associated with the least positive experience.
Methods: Patient experience was assessed using the Inventory to Assess Patient Satisfaction (IAPS), a 22-item questionnaire, completed 7-14 days after the Cytosponge test.
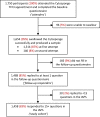
Study cohort: All BEST3 participants who answered ≥ 15 items of the IAPS (N = 1458).
Statistical analysis: A mean IAPS score between 1 and 5 (5 indicates most negative experience) was calculated for each individual. 'Least positive' experience was defined according to the 90th percentile. 167 (11.4%) individuals with a mean IAPS score of ≥ 2.32 were included in the 'least positive' category and compared with the rest of the cohort. Eleven patient characteristics and one procedure-specific factor were assessed as potential predictors of the least positive experience. Multivariable logistic regression analysis using backwards selection was conducted to identify factors independently associated with the least positive experience and with failed swallow at first attempt, one of the strongest predictors of least positive experience.
Results: The majority of responders had a positive experience, with an overall median IAPS score of 1.7 (IQR 1.5-2.1). High (OR = 3.01, 95% CI 2.03-4.46, p < 0.001) or very high (OR = 4.56, 95% CI 2.71-7.66, p < 0.001) anxiety (relative to low/normal anxiety) and a failed swallow at the first attempt (OR = 3.37, 95% CI 2.14-5.30, p < 0.001) were highly significant predictors of the least positive patient experience in multivariable analyses. Additionally, sex (p = 0.036), height (p = 0.032), alcohol intake (p = 0.011) and education level (p = 0.036) were identified as statistically significant predictors.
Conclusion: We have identified factors which predict patient experience. Identifying anxiety ahead of the procedure and discussing particular concerns with patients or giving them tips to help with swallowing the capsule might help improve their experience. Trial registration ISRCTN68382401.
Keywords: Barrett’s oesophagus; Cytosponge; Inventory to assess patient satisfaction; Patient experience.
© 2023. The Author(s).
Conflict of interest statement
RCF is named on patents related to the Cytosponge and related assays that have been licenced to Medtronic by the Medical Research Council. RCF is a shareholder in Cyted Ltd, a company working on early detection technology. PDS reports consulting fees paid to his organisation from GRAIL Inc, outside of the submitted work and being on the advisory committee for NHS England on the use of the Cytosponge (unpaid). The remaining authors have no conflicts of interest to declare.
Figures
References
-
- Oesophageal cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s....
Publication types
MeSH terms
Associated data
Grants and funding
- 27047/CRUK_/Cancer Research UK/United Kingdom
- RG84369/MRC_/Medical Research Council/United Kingdom
- 21047/CRUK_/Cancer Research UK/United Kingdom
- C14478/A21047, C8162/A16892, C8162/A25356, C7492/A17219, C8640/A23385/CRUK_/Cancer Research UK/United Kingdom
- 25356/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Medical