Individual immune cell and cytokine profiles determine platelet-rich plasma composition
- PMID: 36627721
- PMCID: PMC9830842
- DOI: 10.1186/s13075-022-02969-6
Individual immune cell and cytokine profiles determine platelet-rich plasma composition
Abstract
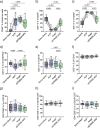
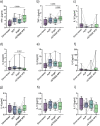
Objective: Platelet-rich plasma (PRP) therapy is increasingly popular to treat musculoskeletal diseases, including tendinopathies and osteoarthritis (OA). To date, it remains unclear to which extent PRP compositions are determined by the immune cell and cytokine profile of individuals or by the preparation method. To investigate this, we compared leukocyte and cytokine distributions of different PRP products to donor blood samples and assessed the effect of pro-inflammatory cytokines on chondrocytes.
Design: For each of three PRP preparations (ACP®, Angel™, and nSTRIDE® APS), products were derived using whole blood samples from twelve healthy donors. The cellular composition of PRP products was analyzed by flow cytometry using DURAClone antibody panels (DURAClone IM Phenotyping Basic and DURAClone IM T Cell Subsets). The MESO QuickPlex SQ 120 system was used to assess cytokine profiles (V-PLEX Proinflammatory Panel 1 Human Kit, Meso Scale Discovery). Primary human chondrocyte 2D and 3D in vitro cultures were exposed to recombinant IFN-γ and TNF-α. Proliferation and chondrogenic differentiation were quantitatively assessed.
Results: All three PRP products showed elevated portions of leukocytes compared to baseline levels in donor blood. Furthermore, the pro-inflammatory cytokines IFN-γ and TNF-α were significantly increased in nSTRIDE® APS samples compared to donor blood and other PRP products. The characteristics of all other cytokines and immune cells from the donor blood, including pro-inflammatory T cell subsets, were maintained in all PRP products. Chondrocyte proliferation was impaired by IFN-γ and enhanced by TNF-α treatment. Differentiation and cartilage formation were compromised upon treatment with both cytokines, resulting in altered messenger ribonucleic acid (mRNA) expression of collagen type 1A1 (COL1A1), COL2A1, and aggrecan (ACAN) as well as reduced proteoglycan content.
Conclusions: Individuals with elevated levels of cells with pro-inflammatory properties maintain these in the final PRP products. The concentration of pro-inflammatory cytokines strongly varies between PRP products. These observations may help to unravel the previously described heterogeneous response to PRP in OA therapy, especially as IFN-γ and TNF-α impacted primary chondrocyte proliferation and their characteristic gene expression profile. Both the individual's immune profile and the concentration method appear to impact the final PRP product.
Trial registration: This study was prospectively registered in the Deutsches Register Klinischer Studien (DRKS) on 4 November 2021 (registration number DRKS00026175).
Keywords: Immune system; Inflammation; Orthobiologics; Osteoarthritis; Regenerative therapies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- FDA. Part 640 - additional standards for human blood and blood products https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?f...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

