Place in therapy of innovative drugs in multiple myeloma in 2021 and 2023 according to an expert panel Delphi consensus
- PMID: 36627863
- PMCID: PMC9616197
- DOI: 10.33393/grhta.2021.2245
Place in therapy of innovative drugs in multiple myeloma in 2021 and 2023 according to an expert panel Delphi consensus
Abstract
Introduction: The objective of this study was to understand the potential use of single agents and drug combinations in multiple myeloma (MM) across treatment lines in the years 2021 and 2023.
Methods: The method used was Delphi Panel Method survey, administered to European Myeloma Network (EMN) Italy Working Group centres. Future treatments were identified assessing all available web-based information sources, including therapies (single drugs or combinations) with strong evidence of efficacy, likely to be on the Italian market in 2021 and 2023. Participants were asked to report on the likelihood of prescription for MM therapies, across treatment lines.
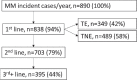
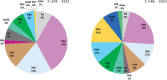
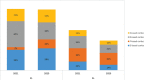
Results: Across the 15 centres taking part in the survey, about 890 patients per year are forecasted to receive a new diagnosis of MM. In 2021, the Panel forecasted 66% of 1L-TE (transplant eligible) patients will be treated with bortezomib-thalidomide-dexamethasone (VTD) and 32% of patients with daratumumab-bortezomib-thalidomide-dexamethasone (DVTd), with a substantial decrease of VTD (15%) and a marked increase of DVTd (81%) forecasted for 2023. The 2L and 3L R(lenalidomide)-based combination treatments are expected to drop and will likely be substituted by a steep increase in P(pomalidomide)-based regimes (from 7% to 23%). On the contrary, in 3L treatment, all combination therapies (with the exception of IsaPd - isatuximab-pomalidomide-dexamethasone) are expected to lose market share in favour of the most recent new therapies.
Conclusions: Expert Panel agrees that many different new drugs and combinations will be used in MM, with different mechanisms of action, both at diagnosis and in subsequent phases of the disease, with a corresponding decline of the drugs currently used.
Keywords: Delphi Panel; European Myeloma Network; Monoclonal antibodies; Multiple myeloma.
Conflict of interest statement
Conflict of interest: M.B. has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb and AbbVie; has served on the advisory boards for Janssen and GSK; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb and Mundipharma. P.B. is a consultant of Certara Italy. S.B. has received honoraria from Celgene, Amgen and Janssen, and Bristol-Myers Squibb; has served on the advisory boards for Celgene, Amgen, Janssen and Karyopharm; has received consultancy fees from Janssen and Takeda. E.Z. has received honoraria from and served on the advisory board for Janssen, BMS, Takeda, Sanofi, Amgen. N.C. has received honoraria from Takeda, Pfizer, Janssen Pharmaceutical, Bristol Myers Squibb, Amgen, Celgene, Novartis. N.G. has received research grants from Celgene, Janssen Pharmaceutical; clinical trial sponsorship from Janssen Pharmaceutical, Millennium Pharmaceutical, GSK; served on the advisory boards for Celgene, Takeda, Janssen Pharmaceutical; and congress fee from Janssen Pharmaceutical, Celgene, Bristol-Myers Squibb. R.Z. has served on the advisory board for Janssen, Celgene, GSK. F.P. has served on the advisory board for Celgene/Bristol-Myers Squibb, Janssen, GSK. V.M. has received honoraria and travel grants from Janssen, Celgene, Bristol-Myers Squibb, Amgen, Takeda. F.D.R. has received honoraria from and served on the advisory boards for Janssen, Celgene, Amgen, Takeda, GSK. M.O. has received honoraria from and served on the advisory boards for Amgen, BMS, Celgene, Janssen, Sanofi, Takeda. M.T.P. has received honoraria from and served on the advisory board for Celgene, Janssen-Cilag; Bristol Myers Squibb, Takeda, Amgen, Sanofi, GSK. P.M. has received personal fees and/or served as a member of advisory boards from/of Amgen, Novartis, BMS, Celgene, Janssen, Sanofi, Gilead, Abbvie, GSK, Jazz, Takeda. The other authors declare no competing conflicts of interest.
Figures





References
LinkOut - more resources
Full Text Sources

