Analisi di budget impact di anakinra nel trattamento della Malattia di Still in Italia
- PMID: 36627970
- PMCID: PMC9677608
- DOI: 10.33393/grhta.2020.2140
Analisi di budget impact di anakinra nel trattamento della Malattia di Still in Italia
Erratum in
-
Erratum in: Budget Impact Analysis of anakinra in the treatment of patients with Still's Disease.Glob Reg Health Technol Assess. 2020 Sep 30;7:91. doi: 10.33393/grhta.2020.2187. eCollection 2020 Jan-Dec. Glob Reg Health Technol Assess. 2020. PMID: 36643911 Free PMC article.
Abstract
Background:: Anakinra, canakinumab and tocilizumab are all effective alternative treatment choice in patients with Still’s disease including both systemic juvenile idiopathic arthritis (SJIA) and adult onset Still’s disease (AOSD).
Objective:: Aim of this study was to estimate the budget impact of the use of anakinra compared to canakinumab and tocilizumab in the treatment of patients with AOSD or SJIA.
Methods:: Considering the perspective of the Italian National Health Service (iNHS), a budget impact model (BIM) was developed to estimate the drug costs of anakinra, canakinumab and tocilizumab up to 12 months. The BIM showed the difference of drug expenditure generated by the base case calculated for current prescription volumes, and for different prescription volume scenarios with increased anakinra prescription. Key variables were tested in the sensitivity analysis.
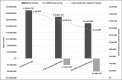
Results:: Compared to the current scenario for SJIA, an increase in the market share of anakinra (40% or 50%) would lead to a reduction in the drug expenditure sustained by iNHS (–€1,087,494 [–12.4%] or –€2,023,990 [–23.1%]). Compared to the current scenario for AOSD, an increase in the market share of anakinra (40% or 50%) would lead to a reduction in the drug expenditure sustained by iNHS (–€4,024,585 [–13.5%] or –€8,049,169 [–27.0%]).
Conclusion:: According to the present analysis, the use of anakinra, as an alternative to canakinumab or tocilizumab in patients with AOSD or SJIA, could represent a cost-saving option for the iNHS.
Conflict of interest statement
Conflict of interest: The Authors declare that they have no conflicts of interest in this research.
Figures
References
-
- Sfriso P, Bindoli S, Galozzi P. Adult-onset Still’s disease: molecular pathophysiology and therapeutic advances. Drugs. 2018;78(12):1187–95. - PubMed
-
- Sfriso P, Priori R, Valesini G et al. Adult onset Still’s disease: an Italian multicenter retrospective observational study of manifestations and treatments in 245 patients. Clin Rheumatol. 2016;35(7):1683–9. - PubMed
LinkOut - more resources
Full Text Sources