(Carbazol-9-ido-κ N)di-chlorido-(η5:η1-2,3,4,5-tetra-methyl-penta-fulvene)tantalum(V)
- PMID: 36628193
- PMCID: PMC9815124
- DOI: 10.1107/S2414314622012019
(Carbazol-9-ido-κ N)di-chlorido-(η5:η1-2,3,4,5-tetra-methyl-penta-fulvene)tantalum(V)
Abstract
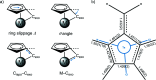
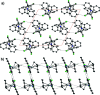
The reaction of (η5:η1-2,3,4,5-tetra-methyl-penta-fulvene)tantalum(V) dicarbazolide chloride (1) with etheric HCl results in the formation of the title compound (2), [Ta(C10H14)(C12H8N)Cl2]. The TaV atom has a distorted tetra-hedral coordination environment in a three-legged piano-stool fashion. The conformation of the penta-fulvene exocyclic C atom to the three other ligands is staggered and not eclipsed, as found in the crystal structure of 1. Inter-molecular inter-actions include π-π stacking, H⋯π inter-actions and weak C-H⋯Cl hydrogen bonds.
Keywords: amide; chloride; crystal structure; pentafulvene; tantalum.
© Graaff et al. 2022.
Figures

References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
-
- Antonelli, D. M., Schaefer, W. P., Parkin, G. & Bercaw, J. E. (1993). J. Organomet. Chem. 462, 213–220.
-
- Beckhaus, R. (2018). Coord. Chem. Rev. 376, 467–477.
-
- Bruker (2016). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
