Taking a chance: How likely am I to receive my preferred treatment in a clinical trial?
- PMID: 36628522
- PMCID: PMC9983058
- DOI: 10.1177/09622802221146305
Taking a chance: How likely am I to receive my preferred treatment in a clinical trial?
Abstract
Researchers should ideally conduct clinical trials under a presumption of clinical equipoise, but in fact trial patients will often prefer one or other of the treatments being compared. Receiving an unblinded preferred treatment may affect the study outcome, possibly beneficially, but receiving a non-preferred treatment may induce 'reluctant acquiescence', and poorer outcomes. Even in blinded trials, patients' primary motivation to enrol may be the chance of potentially receiving a desirable experimental treatment, which is otherwise unavailable. Study designs with a higher probability of receiving a preferred treatment (denoted as 'concordance') will be attractive to potential participants, and investigators, because they may improve recruitment and hence enhance study efficiency. Therefore, it is useful to consider the concordance rates associated with various study designs. We consider this question with a focus on comparing the standard, randomised, two-arm, parallel group design with the two-stage randomised patient preference design and Zelen designs; we also mention the fully randomised and partially randomised patient preference designs. For each of these designs, we evaluate the concordance rate as a function of the proportions randomised to the alternative treatments, the distribution of preferences over treatments, and (for the Zelen designs) the proportion of patients who consent to receive their assigned treatment. We also examine the equity of each design, which we define as the similarity between the concordance rates for participants with different treatment preferences. Finally, we contrast each of the alternative designs with the standard design in terms of gain in concordance and change in equity.
Keywords: Clinical trials; patient recruitment; randomisation; study design; treatment preference.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





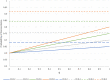
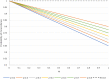


References
-
- Walter SD, Turner R, Macaskill Pet al. et al. Beyond the treatment effect: Evaluating the effects of patient preferences in randomised trials. Stat Methods Med Res 2017; 26: 489–507. - PubMed
-
- Bradley C. Designing medical and educational intervention studies. Diabetes Care 1998; 16: 509–518. - PubMed
-
- Sidani S, Fox M, Epstein D. Conducting a two-stage preference trial: Utility and challenges. Int J Nurs Stud 2015; 52: 1017–1024. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

