Optimizing a therapeutic humanized follicle-stimulating hormone-blocking antibody formulation by protein thermal shift assay
- PMID: 36628526
- PMCID: PMC11658029
- DOI: 10.1111/nyas.14952
Optimizing a therapeutic humanized follicle-stimulating hormone-blocking antibody formulation by protein thermal shift assay
Abstract
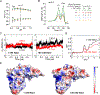
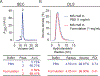
Biopharmaceutical products are formulated using several Food and Drug Administration (FDA) approved excipients within the inactive ingredient limit to maintain their storage stability and shelf life. Here, we have screened and optimized different sets of excipient combinations to yield a thermally stable formulation for the humanized follicle-stimulating hormone (FSH)-blocking antibody, MS-Hu6. We used a protein thermal shift assay in which rising temperatures resulted in the maximal unfolding of the protein at the melting temperature (Tm ). To determine the buffer and pH for a stable solution, four different buffers with a pH range from 3 to 8 were screened. This resulted in maximal Tm s at pH 5.62 for Fab in phosphate buffer and at pH 6.85 for Fc in histidine buffer. Upon testing a range of salt concentrations, MS-Hu6 was found to be more stable at lower concentrations, likely due to reduced hydrophobic effects. Molecular dynamics simulations revealed a higher root-mean-square deviation with 1 mM than with 100 mM salt, indicating enhanced stability, as noted experimentally. Among the stabilizers tested, Tween 20 was found to yield the highest Tm and reversed the salt effect. Among several polyols/sugars, trehalose and sucrose were found to produce higher thermal stabilities. Finally, binding of recombinant human FSH to MS-Hu6 in a final formulation (20 mM phosphate buffer, 1 mM NaCl, 0.001% w/v Tween 20, and 260 mM trehalose) resulted in a thermal shift (increase in Tm ) for the Fab, but expectedly not in the Fc domain. Given that we used a low dose of MS-Hu6 (1 μM), the next challenge would be to determine whether 100-fold higher, industry-standard concentrations are equally stable.
Keywords: FSH; Good Laboratory Practice (GLP); antibody development; biotherapeutics; colloidal stability.
© 2023 New York Academy of Sciences.
Conflict of interest statement
DECLARATION OF COMPETING INTEREST
M.Z. is an inventor on issued patents on inhibiting FSH for the prevention and treatment of osteoporosis and obesity (U.S. Patent 8,435,948 and 11,034,761). M.Z. is also an inventor on pending patent application on composition and use of humanized monoclonal anti–FSH antibodies and is co-inventor of a pending patent on the use of FSH as a target for preventing Alzheimer’s disease. These patents are owned by Icahn School of Medicine at Mount Sinai (ISMMS), and M.Z. would be recipient of royalties,
Figures

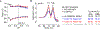


References
-
- Wang W, et al., Antibody structure, instability, and formulation. J Pharm Sci, 2007. 96(1): p. 1–26. - PubMed
-
- Whitaker N, et al., A Formulation Development Approach to Identify and Select Stable Ultra-High-Concentration Monoclonal Antibody Formulations With Reduced Viscosities. J Pharm Sci, 2017. 106(11): p. 3230–3241. - PubMed
-
- Zheng S, et al., Investigating the Degradation Behaviors of a Therapeutic Monoclonal Antibody Associated with pH and Buffer Species. AAPS PharmSciTech, 2017. 18(1): p. 42–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

