Emerging heterologous mRNA-based booster strategies within the COVID-19 vaccine landscape
- PMID: 36629006
- PMCID: PMC9980456
- DOI: 10.1080/21645515.2022.2153532
Emerging heterologous mRNA-based booster strategies within the COVID-19 vaccine landscape
Abstract
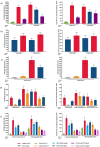
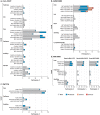
Messenger RNA (mRNA)-based vaccine platforms used for the development of mRNA-1273 and BNT162b2 have provided a robust adaptable approach to offer protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, as variants of concern (VoCs), such as omicron and associated sub-variants, emerge, boosting strategies must also adapt to keep pace with the changing landscape. Heterologous vaccination regimens involving the administration of booster vaccines different than the primary vaccination series offer a practical, effective, and safe approach to continue to reduce the global burden of coronavirus disease 2019 (COVID-19). To understand the immunogenicity, effectiveness, and safety of heterologous mRNA-based vaccination strategies, relevant clinical and real-world observational studies were identified and summarized. Overall, heterologous boosting strategies with mRNA-based vaccines that are currently available and those in development will play an important global role in protecting individuals from COVID-19 caused by emerging VoCs.
Keywords: COVID-19; SARS-CoV-2; heterologous booster; mRNA-1273 vaccine.
Conflict of interest statement
RD, PB, and JMM are employees of Moderna, Inc., and hold stock/stock options. BJK is a consultant for Moderna, Inc., RNH was an employee of Moderna, Inc., at the time of writing the manuscript and held stock/stock options at the company.
Figures

Comment in
-
Heterologous mRNA-based COVID-19 booster strategies: Comment.Hum Vaccin Immunother. 2023 Dec 31;19(1):2174758. doi: 10.1080/21645515.2023.2174758. Epub 2023 Feb 16. Hum Vaccin Immunother. 2023. PMID: 36798966 Free PMC article. No abstract available.
References
-
- World Health Organization . COVID-19 vaccine tracker and landscape; 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
