DNA fragility at the KMT2A/ MLL locus: insights from old and new technologies
- PMID: 36629017
- PMCID: PMC9832561
- DOI: 10.1098/rsob.220232
DNA fragility at the KMT2A/ MLL locus: insights from old and new technologies
Abstract
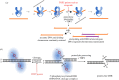
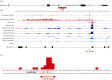
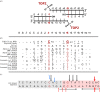
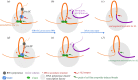
The Mixed-Lineage Leukaemia (MLL/KMT2A) gene is frequently rearranged in childhood and adult acute leukaemia (AL) and in secondary leukaemias occurring after therapy with DNA topoisomerase targeting anti-cancer agents such as etoposide (t-AL). MLL/KMT2A chromosome translocation break sites in AL patients fall within an 8 kb breakpoint cluster region (BCR). Furthermore, MLL/KMT2A break sites in t-AL frequently occur in a much smaller region, or hotspot, towards the 3' end of the BCR, close to the intron 11/exon 12 boundary. These findings have prompted considerable effort to uncover mechanisms behind the apparent fragility of the BCR and particularly the t-AL hotspot. Recent genome-wide analyses have demonstrated etoposide-induced DNA cleavage within the BCR, and it is tempting to conclude that this cleavage explains the distribution of translocation break sites in t-AL. However, the t-AL hotspot and the centre of the observed preferential DNA cleavage are offset by over 250 nucleotides, suggesting additional factors contribute to the distribution of t-AL break sites. We review these recent genomic datasets along with older experimental results, analysis of TOP2 DNA cleavage site preferences and DNA secondary structure features that may lead to break site selection in t-AL MLL/KMT2A translocations.
Keywords: DNA topoisomerase II; KMT2A; MLL; chromosome translocation; etoposide; leukaemia.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

