Metabolically-targeted dCas9 expression in bacteria
- PMID: 36629257
- PMCID: PMC9881133
- DOI: 10.1093/nar/gkac1248
Metabolically-targeted dCas9 expression in bacteria
Abstract
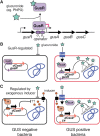
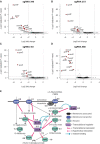
The ability to restrict gene expression to a relevant bacterial species in a complex microbiome is an unsolved problem. In the context of the human microbiome, one desirable target metabolic activity are glucuronide-utilization enzymes (GUS) that are implicated in the toxic re-activation of glucuronidated compounds in the human gastrointestinal (GI) tract, including the chemotherapeutic drug irinotecan. Here, we take advantage of the variable distribution of GUS enzymes in bacteria as a means to distinguish between bacteria with GUS activity, and re-purpose the glucuronide-responsive GusR transcription factor as a biosensor to regulate dCas9 expression in response to glucuronide inducers. We fused the Escherichia coli gusA regulatory region to the dCas9 gene to create pGreg-dCas9, and showed that dCas9 expression is induced by glucuronides, but not other carbon sources. When conjugated from E. coli to Gammaproteobacteria derived from human stool, dCas9 expression from pGreg-dCas9 was restricted to GUS-positive bacteria. dCas9-sgRNAs targeted to gusA specifically down-regulated gus operon transcription in Gammaproteobacteria, with a resulting ∼100-fold decrease in GusA activity. Our data outline a general strategy to re-purpose bacterial transcription factors responsive to exogenous metabolites for precise ligand-dependent expression of genetic tools such as dCas9 in diverse bacterial species.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

