Treatment efficacy in a metastatic small intestinal neuroendocrine tumour grade 2 cohort
- PMID: 36629395
- PMCID: PMC9986391
- DOI: 10.1530/ERC-22-0316
Treatment efficacy in a metastatic small intestinal neuroendocrine tumour grade 2 cohort
Abstract
Small intestinal neuroendocrine tumours (Si-NET) are often studied as a uniform group. Proliferation index Ki-67 influences prognosis and determines tumour grade. We hypothesized that Si-NET grade 2 (G2) tumours, which have a higher Ki-67 than G1 tumours, might benefit less from established treatments for metastatic disease. We conducted a retrospective cohort study of 212 patients with metastatic Si-NET G2 treated in two Swedish hospitals during 20 years (2000-2019). Median cancer-specific survival on first-line somatostatin analogues (SSA) was 77 months. Median progression-free survival (PFS) was 12.4 months when SSA was given as monotherapy and 19 months for all patients receiving first-line SSA. PFS after SSA dose escalation was 6 months in patients with radiological progression. Treatment efficacies of SSA and peptide receptor radionuclide treatment (PRRT) were studied separately in patients with Ki-67 of 3-5%, 5-10% and 10-20%. For SSA, PFS was significantly shorter at higher Ki-67 levels (31, 18 and 10 months, respectively), while there was only a minor difference in PFS for PRRT (29, 25 and 25 months). Median PFS for sequential treatment with interferon-alpha (IFNα), everolimus and chemotherapy was 6, 5 and 9 months. IFNα seemed to be effective in tumours with low somatostatin-receptor expression. In conclusion, established treatments appeared effective in Si-NET G2, despite their higher proliferation index compared to G1 tumours. However, efficacy of SSA but not PRRT was reduced at higher Ki-67 levels. SSA dose escalation provided limited disease stabilization.
Keywords: Ki-67; PRRT; Si-NET; grade 2; interferon; peptide receptor radionuclide treatment; small intestinal neuroendocrine tumours; somatostatin analogues; somatostatin receptor negative.
Conflict of interest statement
The authors declare no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
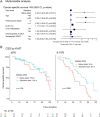

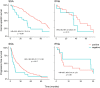

References
-
- Aalbersberg EA, Huizing DMV, Walraven I, Veen der BJde W, Kulkarni HR, Singh A, Stokkel MPM, Baum RP.2019Parameters to predict progression-free and overall survival after peptide receptor radionuclide therapy: a multivariate analysis in 782 patients. Journal of Nuclear Medicine 601259–1265. (10.2967/jnumed.118.224386) - DOI - PubMed
-
- Arnold R, Rinke A, Klose KJ, Müller HH, Wied M, Zamzow K, Schmidt C, Schade-Brittinger C, Barth P, Moll Ret al.2005Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clinical Gastroenterology and Hepatology 3761–771. (10.1016/S1542-3565(0500481-7) - DOI - PubMed
-
- Bertani E, Falconi M, Grana C, Botteri E, Chiappa A, Misitano P, Spada F, Ravizza D, Bazolli B, Fazio N.2015Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: prognostic factors for decision making. International Journal of Surgery (London, England) 2058–64. (10.1016/j.ijsu.2015.06.019) - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

