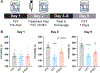5-HT2ARs Mediate Therapeutic Behavioral Effects of Psychedelic Tryptamines
- PMID: 36630260
- PMCID: PMC9939288
- DOI: 10.1021/acschemneuro.2c00718
5-HT2ARs Mediate Therapeutic Behavioral Effects of Psychedelic Tryptamines
Abstract
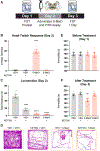
Psychedelic compounds have displayed antidepressant potential in both humans and rodents. Despite their promise, psychedelics can induce undesired effects that pose safety concerns and limit their clinical scalability. The rational development of optimized psychedelic-related medicines will require a full mechanistic understanding of how these molecules produce therapeutic effects. While the hallucinogenic properties of psychedelics are generally attributed to activation of serotonin 2A receptors (5-HT2ARs), it is currently unclear if these receptors also mediate their antidepressant effects as several nonhallucinogenic analogues of psychedelics with antidepressant-like properties have been developed. Moreover, many psychedelics exhibit promiscuous pharmacology, making it challenging to identify their primary therapeutic target(s). Here, we use a combination of pharmacological and genetic tools to demonstrate that activation of 5-HT2A receptors is essential for tryptamine-based psychedelics to produce antidepressant-like effects in rodents. Our results suggest that psychedelic tryptamines can induce hallucinogenic and therapeutic effects through activation of the same receptor.
Keywords: 5-HT2A receptor; 5-methoxy-N,N-dimethyltryptamine; Psychedelic; depression; neuroplasticity; psilocybin; psychoplastogen; structural plasticity.
Figures