Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates
- PMID: 36630502
- PMCID: PMC9833661
- DOI: 10.1126/sciadv.add4623
Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates
Abstract
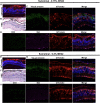
Lipid nanoparticle (LNP)-based mRNA delivery holds promise for the treatment of inherited retinal degenerations. Currently, LNP-mediated mRNA delivery is restricted to the retinal pigment epithelium (RPE) and Müller glia. LNPs must overcome ocular barriers to transfect neuronal cells critical for visual phototransduction, the photoreceptors (PRs). We used a combinatorial M13 bacteriophage-based heptameric peptide phage display library for the mining of peptide ligands that target PRs. We identified the most promising peptide candidates resulting from in vivo biopanning. Dye-conjugated peptides showed rapid localization to the PRs. LNPs decorated with the top-performing peptide ligands delivered mRNA to the PRs, RPE, and Müller glia in mice. This distribution translated to the nonhuman primate eye, wherein robust protein expression was observed in the PRs, Müller glia, and RPE. Overall, we have developed peptide-conjugated LNPs that can enable mRNA delivery to the neural retina, expanding the utility of LNP-mRNA therapies for inherited blindness.
Figures






References
-
- A. V. Garafalo, A. V. Cideciyan, E. Héon, R. Sheplock, A. Pearson, C. WeiYang Yu, A. Sumaroka, G. D. Aguirre, S. G. Jacobson, Progress in treating inherited retinal diseases: Early subretinal gene therapy clinical trials and candidates for future initiatives. Prog. Retin. Eye Res. 77, 100827 (2020). - PMC - PubMed
-
- RetNet–Retinal Information Network (14 February 2022).
-
- S. Makin, Four technologies that could transform the treatment of blindness. Nature 10.1038/d41586-019-01107-8 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

