Influenza Vaccine in Pediatric Recipients of Hematopoietic-Cell Transplants
- PMID: 36630610
- PMCID: PMC11444755
- DOI: 10.1056/NEJMc2210825
Influenza Vaccine in Pediatric Recipients of Hematopoietic-Cell Transplants
Figures
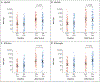
References
-
- GiaQuinta S, Michaels MG, McCullers JA, et al. Randomized, double-blind comparison of standard-dose vs. high-dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatr Transplant 2015;19:219–28. - PubMed
-
- Halasa NB, Savani BN, Asokan I, et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 2016;22:528–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
