Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters
- PMID: 36630611
- PMCID: PMC9847505
- DOI: 10.1056/NEJMc2213948
Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters
Figures
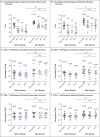
Update of
-
Immunogenicity of the BA.5 Bivalent mRNA Vaccine Boosters.bioRxiv [Preprint]. 2022 Oct 25:2022.10.24.513619. doi: 10.1101/2022.10.24.513619. bioRxiv. 2022. Update in: N Engl J Med. 2023 Feb 9;388(6):565-567. doi: 10.1056/NEJMc2213948. PMID: 36324798 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
