Aromatic micelles: toward a third-generation of micelles
- PMID: 36631075
- PMCID: PMC9851959
- DOI: 10.2183/pjab.99.002
Aromatic micelles: toward a third-generation of micelles
Abstract
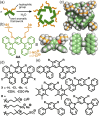
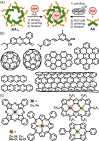
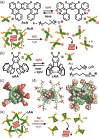
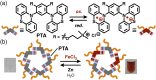
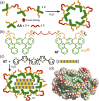
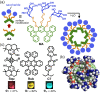
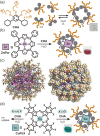
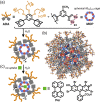
Micelles are useful and widely applied molecular assemblies, formed from amphiphilic molecules, in water. The majority of amphiphiles possess an alkyl chain as the hydrophobic part. Amphiphiles bearing hydrophilic and hydrophobic polymer chains generate so-called polymeric micelles in water. This review focuses on the recent progress of "aromatic micelles", formed from bent polyaromatic/aromatic amphiphiles, for the development of third-generation micelles. Thanks to multiple host-guest interactions, e.g., the hydrophobic effect and π-π/CH-π interactions, the present micelles display wide-ranging uptake abilities toward various hydrophobic compounds in water. In addition to such host functions, new stimuli-responsive aromatic micelles with pH, light, and redox switches, aromatic oligomer micelles, saccharide-coated aromatic micelles, and related cycloalkane-based micelles were recently developed by our group.
Keywords: aromatic micelle; bent aromatic amphiphile; host-guest interaction; stimuli-responsive; water.
Figures


References
-
- Moroi, Y. (1992) Micelles: Theoretical and Applied Aspects. Plenum, New York.
-
- Myers, D. (2006) Surfactant Science and Technology. 3rd ed. Wiley, Hoboken.
-
- Dwars T., Paetzold E., Oehme G. (2005) Reactions in micellar systems. Angew. Chem. Int. Ed. 44, 7174–7199. - PubMed
-
- Alexandridis, P. and Lindman, B. (2000) Amphiphilic Block Copolymers: Self-Assembly and Applications. Elsevier, Amsterdam.
-
- Kataoka K., Harada A., Nagasaki Y. (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 47, 113–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

