Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study
- PMID: 36631153
- PMCID: PMC9832503
- DOI: 10.1136/bmj-2022-072529
Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study
Abstract
Objectives: To determine the clinical sequelae of long covid for a year after infection in patients with mild disease and to evaluate its association with age, sex, SARS-CoV-2 variants, and vaccination status.
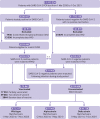
Design: Retrospective nationwide cohort study.
Setting: Electronic medical records from an Israeli nationwide healthcare organisation.
Population: 1 913 234 Maccabi Healthcare Services members of all ages who did a polymerase chain reaction test for SARS-CoV-2 between 1 March 2020 and 1 October 2021.
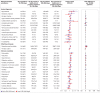
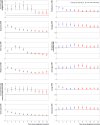
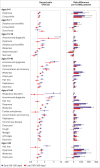
Main outcome measures: Risk of an evidence based list of 70 reported long covid outcomes in unvaccinated patients infected with SARS-CoV-2 matched to uninfected people, adjusted for age and sex and stratified by SARS-CoV-2 variants, and risk in patients with a breakthrough SARS-CoV-2 infection compared with unvaccinated infected controls. Risks were compared using hazard ratios and risk differences per 10 000 patients measured during the early (30-180 days) and late (180-360 days) time periods after infection.
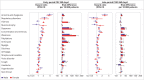
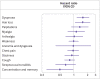
Results: Covid-19 infection was significantly associated with increased risks in early and late periods for anosmia and dysgeusia (hazard ratio 4.59 (95% confidence interval 3.63 to 5.80), risk difference 19.6 (95% confidence interval 16.9 to 22.4) in early period; 2.96 (2.29 to 3.82), 11.0 (8.5 to 13.6) in late period), cognitive impairment (1.85 (1.58 to 2.17), 12.8, (9.6 to 16.1); 1.69 (1.45 to 1.96), 13.3 (9.4 to 17.3)), dyspnoea (1.79 (1.68 to 1.90), 85.7 (76.9 to 94.5); 1.30 (1.22 to 1.38), 35.4 (26.3 to 44.6)), weakness (1.78 (1.69 to 1.88), 108.5, 98.4 to 118.6; 1.30 (1.22 to 1.37), 50.2 (39.4 to 61.1)), and palpitations (1.49 (1.35 to 1.64), 22.1 (16.8 to 27.4); 1.16 (1.05 to 1.27), 8.3 (2.4 to 14.1)) and with significant but lower excess risk for streptococcal tonsillitis and dizziness. Hair loss, chest pain, cough, myalgia, and respiratory disorders were significantly increased only during the early phase. Male and female patients showed minor differences, and children had fewer outcomes than adults during the early phase of covid-19, which mostly resolved in the late period. Findings remained consistent across SARS-CoV-2 variants. Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnoea and similar risk for other outcomes compared with unvaccinated infected patients.
Conclusions: This nationwide study suggests that patients with mild covid-19 are at risk for a small number of health outcomes, most of which are resolved within a year from diagnosis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- World Health Organization. Coronavirus Disease WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
-
- World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants.
-
- Centers for Disease Control and Prevention. COVID-19: Variants of the Virus. 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant.html.
-
- World Health Organization. Coronavirus disease (COVID-19). https://www.who.int/health-topics/coronavirus.
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
