The beneficial effects of chick embryo extract preconditioning on hair follicle stem cells: A promising strategy to generate Schwann cells
- PMID: 36631409
- PMCID: PMC10334277
- DOI: 10.1111/cpr.13397
The beneficial effects of chick embryo extract preconditioning on hair follicle stem cells: A promising strategy to generate Schwann cells
Abstract
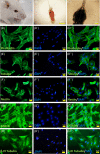
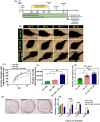
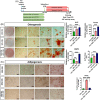
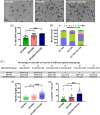
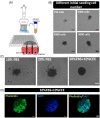
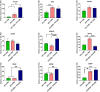
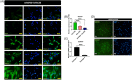
The beneficial effects of hair follicle stem cells in different animal models of nervous system conditions have been extensively studied. While chick embryo extract (CEE) has been used as a growth medium supplement for these stem cells, this is the first study to show the effect of CEE on them. The rat hair follicle stem cells were isolated and supplemented with 10% fetal bovine serum plus 10% CEE. The migration rate, proliferative capacity and multipotency were evaluated along with morphometric alteration and differentiation direction. The proteome analysis of CEE content identified effective factors of CEE that probably regulate fate and function of stem cells. The CEE enhances the migration rate of stem cells from explanted bulges as well as their proliferation, likely due to activation of AP-1 and translationally controlled tumour protein (TCTP) by thioredoxin found in CEE. The increased length of outgrowth may be the result of cyclic AMP response element binding protein (CREB) phosphorylation triggered by active CamKII contained in CEE. Further, CEE supplementation upregulates the expression of vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. The elevated expression of target genes and proteins may be due to CREB, AP-1 and c-Myc activation in these stem cells. Given the increased transcript levels of neurotrophins, VEGF, and the expression of PDGFR-α, S100B, MBP and SOX-10 protein, it is possible that CEE promotes the fate of these stem cells towards Schwann cells.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Sieber‐Blum M, Hu Y. Epidermal neural crest stem cells (EPI‐NCSC) and pluripotency. Stem Cell Rev. 2008;4(4):256‐260. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

