FRMD3 inhibits the growth and metastasis of breast cancer through the ubiquitination-mediated degradation of vimentin and subsequent impairment of focal adhesion
- PMID: 36631457
- PMCID: PMC9834407
- DOI: 10.1038/s41419-023-05552-2
FRMD3 inhibits the growth and metastasis of breast cancer through the ubiquitination-mediated degradation of vimentin and subsequent impairment of focal adhesion
Abstract
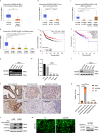
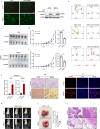
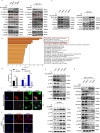
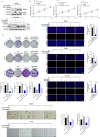
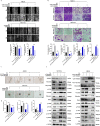
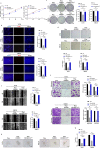
Recurrence and metastasis are the main causes of breast cancer (BRCA)-related death and remain a challenge for treatment. In-depth research on the molecular mechanisms underlying BRCA progression has been an important basis for developing precise biomarkers and therapy targets for early prediction and treatment of progressed BRCA. Herein, we identified FERM domain-containing protein 3 (FRMD3) as a novel potent BRCA tumor suppressor which is significantly downregulated in BRCA clinical tissue and cell lines, and low FRMD3 expression has been closely associated with progressive BRCA and shortened survival time in BRCA patients. Overexpression and knockdown experiments have revealed that FRMD3 significantly inhibits BRCA cell proliferation, migration, and invasion in vitro and suppresses BRCA xenograft growth and metastasis in vivo as well. Mechanistically, FRMD3 can interact with vimentin and ubiquitin protein ligase E3A(UBE3A) to induce the polyubiquitin-mediated proteasomal degradation of vimentin, which subsequently downregulates focal adhesion complex proteins and pro-cancerous signaling activation, thereby resulting in cytoskeletal rearrangement and defects in cell morphology and focal adhesion. Further evidence has confirmed that FRMD3-mediated vimentin degradation accounts for the anti-proliferation and anti-metastasis effects of FRMD3 on BRCA. Moreover, the N-terminal ubiquitin-like domain of FRMD3 has been identified as responsible for FRMD3-vimentin interaction through binding the head domain of vimentin and the truncated FRMD3 with the deletion of ubiquitin-like domain almost completely loses the anti-BRCA effects. Taken together, our study indicates significant potential for the use of FRMD3 as a novel prognosis biomarker and a therapeutic target of BRCA and provides an additional mechanism underlying the degradation of vimentin and BRCA progression.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A tumor suppressor protein encoded by circKEAP1 inhibits osteosarcoma cell stemness and metastasis by promoting vimentin proteasome degradation and activating anti-tumor immunity.J Exp Clin Cancer Res. 2024 Feb 21;43(1):52. doi: 10.1186/s13046-024-02971-7. J Exp Clin Cancer Res. 2024. PMID: 38383479 Free PMC article.
-
RNF144A functions as a tumor suppressor in breast cancer through ubiquitin ligase activity-dependent regulation of stability and oncogenic functions of HSPA2.Cell Death Differ. 2020 Mar;27(3):1105-1118. doi: 10.1038/s41418-019-0400-z. Epub 2019 Aug 13. Cell Death Differ. 2020. PMID: 31406303 Free PMC article.
-
TRIM47 promotes hypopharyngeal and laryngeal cancers progression through promoting K63-linked ubiquitination of vimentin.Cancer Sci. 2025 Feb;116(2):367-380. doi: 10.1111/cas.16397. Epub 2024 Nov 25. Cancer Sci. 2025. PMID: 39584529 Free PMC article.
-
BCAS3 exhibits oncogenic properties by promoting CRL4A-mediated ubiquitination of p53 in breast cancer.Cell Prolif. 2021 Aug;54(8):e13088. doi: 10.1111/cpr.13088. Epub 2021 Jul 9. Cell Prolif. 2021. PMID: 34240781 Free PMC article.
-
CCDC65 as a new potential tumor suppressor induced by metformin inhibits activation of AKT1 via ubiquitination of ENO1 in gastric cancer.Theranostics. 2021 Jul 13;11(16):8112-8128. doi: 10.7150/thno.54961. eCollection 2021. Theranostics. 2021. PMID: 34335983 Free PMC article.
Cited by
-
A Novel Role for FERM Domain-Containing Protein 3 in CKD.Kidney360. 2024 Dec 1;5(12):1799-1812. doi: 10.34067/KID.0000000602. Epub 2024 Oct 16. Kidney360. 2024. PMID: 39450948 Free PMC article.
-
UCHL3: a crucial deubiquitinase in DNA damage repair and tumor progression.Cancer Cell Int. 2025 Jul 21;25(1):276. doi: 10.1186/s12935-025-03884-x. Cancer Cell Int. 2025. PMID: 40691601 Free PMC article. Review.
-
A tumor suppressor protein encoded by circKEAP1 inhibits osteosarcoma cell stemness and metastasis by promoting vimentin proteasome degradation and activating anti-tumor immunity.J Exp Clin Cancer Res. 2024 Feb 21;43(1):52. doi: 10.1186/s13046-024-02971-7. J Exp Clin Cancer Res. 2024. PMID: 38383479 Free PMC article.
-
Exploring vimentin's role in breast cancer via PICK1 alternative polyadenylation and the miR-615-3p-PICK1 interaction.Biofactors. 2025 Jan-Feb;51(1):e2147. doi: 10.1002/biof.2147. Biofactors. 2025. PMID: 39781570 Free PMC article.
-
METTL16 regulates the mRNA stability of FBXO5 via m6A modification to facilitate the malignant behavior of breast cancer.Cancer Metab. 2024 Jul 25;12(1):22. doi: 10.1186/s40170-024-00351-5. Cancer Metab. 2024. PMID: 39061113 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

