Preclinical evaluation of FLT190, a liver-directed AAV gene therapy for Fabry disease
- PMID: 36631545
- PMCID: PMC10284695
- DOI: 10.1038/s41434-022-00381-y
Preclinical evaluation of FLT190, a liver-directed AAV gene therapy for Fabry disease
Abstract
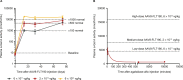
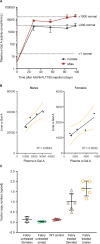
Fabry disease is an X-linked lysosomal storage disorder caused by loss of alpha-galactosidase A (α-Gal A) activity and is characterized by progressive accumulation of glycosphingolipids in multiple cells and tissues. FLT190, an investigational gene therapy, is currently being evaluated in a Phase 1/2 clinical trial in patients with Fabry disease (NCT04040049). FLT190 consists of a potent, synthetic capsid (AAVS3) containing an expression cassette with a codon-optimized human GLA cDNA under the control of a liver-specific promoter FRE1 (AAV2/S3-FRE1-GLAco). For mouse studies FLT190 genome was pseudotyped with AAV8 for efficient transduction. Preclinical studies in a murine model of Fabry disease (Gla-deficient mice), and non-human primates (NHPs) showed dose-dependent increases in plasma α-Gal A with steady-state observed 2 weeks following a single intravenous dose. In Fabry mice, AAV8-FLT190 treatment resulted in clearance of globotriaosylceramide (Gb3) and globotriaosylsphingosine (lyso-Gb3) in plasma, urine, kidney, and heart; electron microscopy analyses confirmed reductions in storage inclusion bodies in kidney and heart. In NHPs, α-Gal A expression was consistent with the levels of hGLA mRNA in liver, and no FLT190-related toxicities or adverse events were observed. Taken together, these studies demonstrate preclinical proof-of-concept of liver-directed gene therapy with FLT190 for the treatment of Fabry disease.
© 2023. The Author(s).
Conflict of interest statement
JJ, AK, LT, EC, NC, DV, PH, PK, CC, CM, MC, JK, RS, RC are employees and/or shareholders of Freeline Therapeutics. AN is a Director, shareholder, and holds patents with Freeline Therapeutics. DH: Advisory Boards: Freeline Therapeutics, Sanofi, Takeda, Amicus, Idorsia; Consulting Fees: Freeline Therapeutics, Sanofi, Takeda, Amicus, Idorsia, Protalix, Sangamo; Honoraria: Freeline Therapeutics, Sanofi, Takeda, Amicus, Idorsia. The other authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

