Antibody-drug conjugates in lung cancer: dawn of a new era?
- PMID: 36631624
- PMCID: PMC9834242
- DOI: 10.1038/s41698-022-00338-9
Antibody-drug conjugates in lung cancer: dawn of a new era?
Abstract
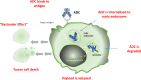
Antibody-drug conjugates (ADCs) are one of fastest growing classes of oncology drugs in modern drug development. By harnessing the powers of both cytotoxic chemotherapy and targeted therapy, ADCs are unique in offering the potential to deliver highly potent cytotoxic agents to cancer cells which express a pre-defined cell surface target. In lung cancer, the treatment paradigm has shifted dramatically in recent years, and now ADCs are now joining the list as potential options for lung cancer patients. Since 2020, the first ADC for NSCLC patients has been FDA-approved (trastuzumab deruxtecan) and two ADCs have been granted FDA Breakthrough Therapy Designation, currently under evaluation (patritumab deruxtecan, telisotuzumab vedotin). Furthermore, several early-phase trials are assessing various novel ADCs, either as monotherapy or in combinations with advanced lung cancer, and more selective and potent ADCs are expected to become therapeutic options in clinic soon. In this review, we discuss the structure and mechanism of action of ADCs, including insights from pre-clinical work; we summarize the ADCs' recent progress in lung cancer, describe toxicity profiles of ADCs, and explore strategies designed to enhance ADC potency and overcome resistance. In addition, we discuss novel ADC strategies of interest in lung cancer, including non-cytotoxic payloads, such as immunomodulatory and anti-apoptotic agents.
© 2023. The Author(s).
Conflict of interest statement
X.L. receives consulting/advisory fees from EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Novartis, Eli Lilly, Boehringer Ingelheim, Hengrui Therapeutics, Janssen, Abbvie, and Research Funding from Eli Lilly, EMD Serono, Regeneron, and Boehringer Ingelheim. T.Y. declares the following: Grant/Research Support (to institution); Acrivon, Artios, AstraZeneca, Bayer, Beigene, BioNTech, Blueprint, BMS, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, Haihe, ImmuneSensor, Ionis, Ipsen, Jounce, Karyopharm, K.S.Q., Kyowa, Merck, Mirati, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Rubius, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, Vivace and Zenith. Consultancies: AbbVie, AstraZeneca, Acrivon, Adagene, Almac, Aduro, Amphista, Artios, Athena, Atrin, Avoro, Axiom, Baptist Health Systems, Bayer, Beigene, Boxer, Bristol Myers Squibb, C4 Therapeutics, Calithera, Cancer Research UK, Clovis, Cybrexa, Diffusion, EMD Serono, F-Star, Genmab, Glenmark, GLG, Globe Life Sciences, GSK, Guidepoint, Idience, Ignyta, I-Mab, ImmuneSensor, Institut Gustave Roussy, Intellisphere, Jansen, Kyn, MEI pharma, Mereo, Merck, Natera, Nexys, Novocure, OHSU, OncoSec, Ono Pharma, Pegascy, PER, Pfizer, Piper-Sandler, Prolynx, Repare, resTORbio, Roche, Schrodinger, Theragnostics, Varian, Versant, Vibliome, Xinthera, Zai Labs and ZielBio; stockholder in Seagen. J.V.H. declares the following: Stock and Other Ownership Interests: Cardinal Spine, Bio-Tree; Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Spectrum; Pharmaceuticals, Guardant Health, Hengrui Pharmaceutical, GlaxoSmithKline, EMD Serono, Lilly, Takeda, Sanofi/Aventis, Genentech/Roche, Boehringer Ingelheim, Catalyst Biotech, Foundation medicine, Novartis, Mirati Therapeutics, BrightPath Biotheraputics, Janssen, Nexus Health Systems, Pneuma Respiratory, Kairos Ventures, Roche, Leads Biolabs; Research Funding: AstraZeneca (Inst), Spectrum Pharmaceuticals, GlaxoSmithKline; Patents, Royalties, Other Intellectual Property: Licensing agreement between Spectrum and MD Anderson (including myself) regarding intellectual property for treatment of EGFR and HER2 exon 20 mutations. F.M.B. declares the following; Consulting: AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., Genentech Inc., IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks; Advisory Committee: Black Diamond, Biovica, Eisai, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, Zentalis; Sponsored Research (to the institution): Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., Taiho Pharmaceutical Co. N.C. reports no conflicts of interest.
Figures

References
-
- Tolcher A. W. The evolution of antibody-drug conjugates: a positive inflexion point. Am Soc Clin Oncol Educ B. 40, 1–8 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

