An electric molecular motor
- PMID: 36631649
- PMCID: PMC9834048
- DOI: 10.1038/s41586-022-05421-6
An electric molecular motor
Abstract
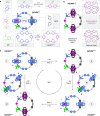
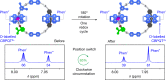
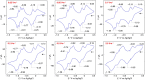
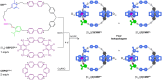
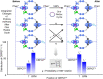
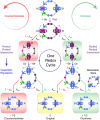
Macroscopic electric motors continue to have a large impact on almost every aspect of modern society. Consequently, the effort towards developing molecular motors1-3 that can be driven by electricity could not be more timely. Here we describe an electric molecular motor based on a [3]catenane4,5, in which two cyclobis(paraquat-p-phenylene)6 (CBPQT4+) rings are powered by electricity in solution to circumrotate unidirectionally around a 50-membered loop. The constitution of the loop ensures that both rings undergo highly (85%) unidirectional movement under the guidance of a flashing energy ratchet7,8, whereas the interactions between the two rings give rise to a two-dimensional potential energy surface (PES) similar to that shown by FOF1 ATP synthase9. The unidirectionality is powered by an oscillating10 voltage11,12 or external modulation of the redox potential13. Initially, we focused our attention on the homologous [2]catenane, only to find that the kinetic asymmetry was insufficient to support unidirectional movement of the sole ring. Accordingly, we incorporated a second CBPQT4+ ring to provide further symmetry breaking by interactions between the two mobile rings. This demonstration of electrically driven continual circumrotatory motion of two rings around a loop in a [3]catenane is free from the production of waste products and represents an important step towards surface-bound14 electric molecular motors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

