Effects of low-doses of methamphetamine on d-fenfluramine-induced head-twitch response (HTR) in mice during ageing and c-fos expression in the prefrontal cortex
- PMID: 36631757
- PMCID: PMC9835290
- DOI: 10.1186/s12868-022-00766-0
Effects of low-doses of methamphetamine on d-fenfluramine-induced head-twitch response (HTR) in mice during ageing and c-fos expression in the prefrontal cortex
Abstract
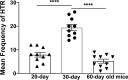
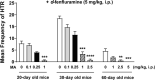
Background: The head-twitch response (HTR) in mice is considered a behavioral model for hallucinogens and serotonin 5-HT2A receptor function, as well as Tourette syndrome in humans. It is mediated by 5-HT2A receptor agonists such as ( ±)- 2,5-dimethoxy-4-iodoamphetamine (DOI) in the prefrontal cortex (PFC). The 5-HT2A antagonist EMD 281014, can prevent both DOI-induced HTR during ageing and c-fos expression in different regions of PFC. Moreover, the nonselective monoamine releaser methamphetamine (MA) suppressed DOI-induced HTR through ageing via concomitant activation of inhibitory 5-HT1A receptors, but enhanced DOI-evoked c-fos expression. d-Fenfluramine is a selective 5-HT releaser and induces HTR in mice, whereas MA does not. Currently, we investigated whether EMD 281014 or MA would alter: (1) d-fenfluramine-induced HTR frequency in 20-, 30- and 60-day old mice, (2) d-fenfluramine-evoked c-fos expression in PFC, and (3) whether blockade of inhibitory serotonergic 5-HT1A- or adrenergic ɑ2-receptors would prevent suppressive effect of MA on d-fenfluramine-induced HTR.
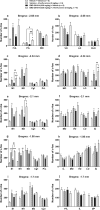
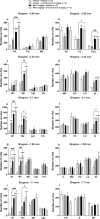
Results: EMD 281014 (0.001-0.05 mg/kg) or MA (0.1-5 mg/kg) blocked d-fenfluramine-induced HTR dose-dependently during ageing. The 5-HT1A antagonist WAY 100635 countered the inhibitory effect of MA on d-fenfluramine-induced HTR in 30-day old mice, whereas the adrenergic ɑ2 antagonist RS 79948 reversed MA's inhibitory effect in both 20- and 30- day old mice. d-Fenfluramine significantly increased c-fos expressions in PFC regions. MA (1 mg/kg) pretreatment significantly increased d-fenfluramine-evoked c-fos expression in different regions of PFC. EMD 281014 (0.05 mg/kg) failed to prevent d-fenfluramine-induced c-fos expression, but significantly increased it in one PFC region (PrL at - 2.68 mm).
Conclusion: EMD 281014 suppressed d-fenfluramine-induced HTR but failed to prevent d-fenfluramine-evoked c-fos expression which suggest involvement of additional serotonergic receptors in the mediation of evoked c-fos. The suppressive effect of MA on d-fenfluramine-evoked HTR is due to well-recognized functional interactions between stimulatory 5-HT2A- and the inhibitory 5-HT1A- and ɑ2-receptors. MA-evoked increases in c-fos expression in PFC regions are due to the activation of diverse monoaminergic receptors through increased synaptic concentrations of 5-HT, NE and/or DA, which may also account for the additive effect of MA on d-fenfluramine-evoked changes in c-fos expression. Our findings suggest potential drug receptor functional interaction during development when used in combination.
Keywords: 5-HT1A receptor; 5-HT2A receptor; Adrenergic ɑ2-receptors; Head-twitch response; Methamphetamine; d-fenfluramine.
© 2023. The Author(s).
Conflict of interest statement
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
An ontogenic study of receptor mechanisms by which acute administration of low-doses of methamphetamine suppresses DOI-induced 5-HT2A-receptor mediated head-twitch response in mice.BMC Neurosci. 2022 Jan 4;23(1):2. doi: 10.1186/s12868-021-00686-5. BMC Neurosci. 2022. PMID: 34983399 Free PMC article.
-
Ultra-low doses of methamphetamine suppress 5-hydroxytryptophan-induced head-twitch response in mice during aging.Behav Pharmacol. 2024 Oct 1;35(7):367-377. doi: 10.1097/FBP.0000000000000789. Epub 2024 Aug 5. Behav Pharmacol. 2024. PMID: 39206775
-
Long-lasting alterations in 5-HT2A receptor after a binge regimen of methamphetamine in mice.Int J Neuropsychopharmacol. 2014 Oct;17(10):1647-58. doi: 10.1017/S1461145714000455. Epub 2014 Apr 24. Int J Neuropsychopharmacol. 2014. PMID: 24763081
-
[Serotonergic control of prefrontal cortex].Rev Neurol. 2004 Sep 16-30;39(6):539-47. Rev Neurol. 2004. PMID: 15467993 Review. Spanish.
-
Serotonin and prefrontal cortex function: neurons, networks, and circuits.Mol Neurobiol. 2011 Dec;44(3):449-64. doi: 10.1007/s12035-011-8214-0. Epub 2011 Nov 11. Mol Neurobiol. 2011. PMID: 22076606 Free PMC article. Review.
References
-
- Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933. doi: 10.1016/j.neuropharm.2019.107933. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

