Phosphate, calcium, and vitamin D signaling, transport, and metabolism in the endometria of cyclic ewes
- PMID: 36631878
- PMCID: PMC9835233
- DOI: 10.1186/s40104-022-00803-2
Phosphate, calcium, and vitamin D signaling, transport, and metabolism in the endometria of cyclic ewes
Abstract
Background: Recent evidence suggests important roles for progesterone (P4) and interferon tau in the regulation of calcium, phosphate, and vitamin D signaling in the uteri of pregnant sheep. However, the effects of P4 and estradiol (E2), with respect to the expression of their receptors PGR and ESR1, respectively, in uterine epithelia on mineral signaling during the estrous cycle has not been investigated. Estrous cycles of mature Suffolk ewes were synchronized, prostaglandin F2α was administered, and ewes were observed for estrus (designated as Day 0) in the presence of vasectomized rams. On Days 1, 9, or 14 of the estrous cycle, hysterectomies were performed.
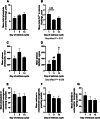
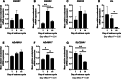
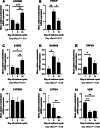
Results: 25-hydroxyvitamin D was more abundant in plasma from ewes on Day 14 than Day 1 (P < 0.05). Expression of fibroblast growth factor receptor 2 (FGFR2), a disintegrin and metalloprotease 17 (ADAM17), and parathyroid hormone-related protein (PTHrP) mRNAs was greater in endometria on Day 9 compared to Days 1 and 14 (P < 0.01). Similarly, expression of transient receptor potential cation channel subfamily V member 6 (TRPV6) mRNA was greater in endometria on Day 9 than Day 1 (P < 0.05). ATPase plasma membrane Ca2+ transporting 4 (ATP2B4) and S100 calcium binding protein G (S100G) mRNA expression was greater in endometria on Day 14 than on Days 1 and 9 (P < 0.01). In contrast, endometrial expression of vitamin D receptor (VDR) mRNA was lower on Days 9 and 14 than Day 1 (P < 0.01). Expression of klotho (KL) (P < 0.05) and cytochrome P450 family 24 subfamily A member 1 (CYP24) (P < 0.01) mRNAs was lower on Day 14 than Days 1 and 9. ADAM17, FGF23, CYP2R1, CYP27B1, KL, and VDR proteins immunolocalized to the uterine myometrium, blood vessels, and uterine luminal (LE), superficial glandular (sGE), and glandular (GE) epithelia. S100A9 protein was weakly expressed in the uterine myometrium, LE, sGE, and GE. Immunoreactivity of CYP2R1 and KL proteins in uterine LE and sGE was less on Day 1 than on Days 9 and 14. In contrast, S100G protein was expressed exclusively by GE, and immunoreactive S100G protein was less on Day 9. S100A12 protein localized to stromal cells of the uterine stratum spongiosum and blood vessels, but not by uterine epithelial cells.
Conclusion: Collectively, these results implicate E2, P4, and PGR in the regulation of phosphate, calcium, and vitamin D signaling in cyclic ewes.
Keywords: Calcium; Endometrium; Ovine; Phosphate; Vitamin D.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Effects of exogenous progesterone on the expression of mineral regulatory molecules by ovine endometrium and placentomes†.Biol Reprod. 2022 Jun 13;106(6):1126-1142. doi: 10.1093/biolre/ioac042. Biol Reprod. 2022. PMID: 35191486 Free PMC article.
-
Effects of progesterone and interferon tau on ovine endometrial phosphate, calcium, and vitamin D signaling†.Biol Reprod. 2022 May 17;106(5):888-899. doi: 10.1093/biolre/ioac027. Biol Reprod. 2022. PMID: 35134855 Free PMC article.
-
Novel mineral regulatory pathways in ovine pregnancy: II. Calcium-binding proteins, calcium transporters, and vitamin D signaling.Biol Reprod. 2021 Jul 2;105(1):232-243. doi: 10.1093/biolre/ioab063. Biol Reprod. 2021. PMID: 33822885
-
Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways.Reprod Biol. 2008 Nov;8(3):179-211. doi: 10.1016/s1642-431x(12)60012-6. Reprod Biol. 2008. PMID: 19092983 Review.
-
Biology of progesterone action during pregnancy recognition and maintenance of pregnancy.Front Biosci. 2002 Sep 1;7:d1879-98. doi: 10.2741/spencer. Front Biosci. 2002. PMID: 12161340 Review.
Cited by
-
Progesterone regulates tissue non-specific alkaline phosphatase (TNSALP) expression and activity in ovine utero-placental tissues.J Anim Sci Biotechnol. 2024 Jul 3;15(1):90. doi: 10.1186/s40104-024-01048-x. J Anim Sci Biotechnol. 2024. PMID: 38956701 Free PMC article.
-
Early Embryonic Development in Agriculturally Important Species.Animals (Basel). 2024 Jun 26;14(13):1882. doi: 10.3390/ani14131882. Animals (Basel). 2024. PMID: 38997994 Free PMC article. Review.
-
CREB1 Is Involved in miR-134-5p-Mediated Endometrial Stromal Cell Proliferation, Apoptosis, and Autophagy.Cells. 2023 Oct 31;12(21):2554. doi: 10.3390/cells12212554. Cells. 2023. PMID: 37947633 Free PMC article.
-
Characterization of the expression of XPR1 in ovine utero-placental tissues.Reproduction. 2025 May 13;169(6):e250072. doi: 10.1530/REP-25-0072. Print 2025 Jun 1. Reproduction. 2025. PMID: 40310868 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

