Chinese herbal medicine for drug-induced liver injury in patients with HIV/AIDS: A systematic review of randomized controlled trials
- PMID: 36632130
- PMCID: PMC9826828
- DOI: 10.1016/j.imr.2022.100918
Chinese herbal medicine for drug-induced liver injury in patients with HIV/AIDS: A systematic review of randomized controlled trials
Abstract
Background: To explore the effectiveness and safety of Chinese herbal medicine (CHM) for drug-induced liver injury (DILI) in patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS).
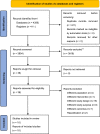
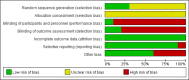
Methods: A systematic search was made of eight databases (Pubmed, Cochrane Library, Web of Science, Embase, CNKI, Wanfang, VIP, Sinomed) and two trial registries (WHO ICTRP, ClinicalTrials.gov) from inception to September 2022. The effect size was presented as risk ratio (RR) or mean difference (MD) with their 95% confidence interval (CI). The Cochrane Risk of Bias and Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tools were used for quality appraisal.
Results: Ten randomized controlled trials (RCTs) involving 732 participants were included. Comparing CHM alone with routine treatment, the CHM group showed lower aspartate aminotransferase (MD=-11.47 U/L, 95%CI[-13.05, -9.89], low certainty), lower alanine aminotransferase (MD=-2.68 U/L, 95%CI[-4.27, -1.08], low certainty), lower total bilirubin (MD=-4.31 mmol/L, 95%CI[-5.66, -2.96], low certainty), lower bilirubin direct (MD=-3.19 mmol/L, 95%CI[-3.87, -2.51], low certainty), and higher effective rate (assessed by symptoms and liver indicators) (RR=1.13, 95%CI[1.06, 1.20], low certainty). A significant difference was also found in CHM plus routine treatment versus routine treatment in the previous outcomes. No significant difference was found on helper T cells among these comparisons. Only one RCT reported safety of CHM and found no adverse reaction during the trial.
Conclusions: CHM may improve the liver function indices and effective rate for HIV/AIDS patients with DILI. However, the sample size was small and quality was low. Larger-samples of high-quality trials are needed.
Keywords: Acquired immunodeficiency syndrome; Chinese herbal medicine; Drug-induced liver injury; Human immunodeficiency virus; Systematic review.
© 2023 Korea Institute of Oriental Medicine. Published by Elsevier B.V.
Figures






Similar articles
-
Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD006404. doi: 10.1002/14651858.CD006404.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD006404. doi: 10.1002/14651858.CD006404.pub4. PMID: 30620055 Free PMC article. Updated.
-
Chinese herbal medicine for post-viral fatigue: A systematic review of randomized controlled trials.PLoS One. 2024 Mar 21;19(3):e0300896. doi: 10.1371/journal.pone.0300896. eCollection 2024. PLoS One. 2024. PMID: 38512808 Free PMC article.
-
Exploring the role of Chinese herbal medicine in the long-term management of postoperative ovarian endometriotic cysts: a systematic review and meta-analysis.Front Pharmacol. 2024 Jun 7;15:1376037. doi: 10.3389/fphar.2024.1376037. eCollection 2024. Front Pharmacol. 2024. PMID: 38910886 Free PMC article.
-
The efficacy and safety of Chinese herbal medicine as an add-on therapy for type 2 diabetes mellitus patients with carotid atherosclerosis: An updated meta-analysis of 27 randomized controlled trials.Front Pharmacol. 2023 Mar 23;14:1091718. doi: 10.3389/fphar.2023.1091718. eCollection 2023. Front Pharmacol. 2023. PMID: 37033624 Free PMC article.
-
Efficacy and safety of Chinese herbal medicine for atopic dermatitis: Evidence from eight high-quality randomized placebo-controlled trials.Front Pharmacol. 2022 Sep 27;13:927304. doi: 10.3389/fphar.2022.927304. eCollection 2022. Front Pharmacol. 2022. PMID: 36238577 Free PMC article.
Cited by
-
Bird's eye view of natural products for the development of new anti-HIV agents: Understanding from a therapeutic viewpoint.Animal Model Exp Med. 2025 Mar;8(3):441-457. doi: 10.1002/ame2.12563. Epub 2025 Feb 7. Animal Model Exp Med. 2025. PMID: 39921221 Free PMC article. Review.
References
-
- HIV.gov . 2020. Overview: About HIV & AIDS: What Are HIV and AIDS?https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/what-are-hiv-... Published. Accessed 26 March 2021.
-
- World Health Organization . 2019. HIV/AIDS.https://www.who.int/news-room/fact-sheets/detail/hiv-aids Published. Accessed 3 June 2021.
-
- CDC.gov . 2020. HIV Basics.https://www.cdc.gov/hiv/basics/index.html Published. Accessed 26 March 2021.
-
- GBD 2017 HIV collaborators Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6(12):e831–e859. - PMC - PubMed
-
- Gervasoni C., Cattaneo D., Filice C., Galli M. Drug-induced liver steatosis in patients with HIV infection. Pharmacol Res. 2019;145 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

