Modelling KNDy neurons and gonadotropin-releasing hormone pulse generation
- PMID: 36632147
- PMCID: PMC9823092
- DOI: 10.1016/j.coemr.2022.100407
Modelling KNDy neurons and gonadotropin-releasing hormone pulse generation
Abstract
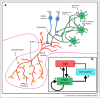
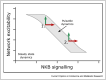
The pulsatile release of gonadotropin-releasing hormone (GnRH) and its frequency are crucial for healthy reproductive function. To understand what drives GnRH pulses, a combination of experimental and mathematical modelling approaches has been used. Early work focussed on the possibility that GnRH pulse generation is an intrinsic feature of GnRH neurons, with autocrine feedback generating pulsatility. However, there is now ample evidence suggesting that a network of upstream neurons secreting kisspeptin, neurokinin-B and dynorphin are the source of this GnRH pulse generator. The interplay of slow positive and negative feedback via neurokinin-B and dynorphin, respectively, allows the network to act as a relaxation oscillator, driving pulsatile secretion of kisspeptin, and consequently, of GnRH and LH. Here, we review the mathematical modelling approaches exploring both scenarios and suggest that with pulsatile GnRH secretion driven by the KNDy pulse generator, autocrine feedback still has the potential to modulate GnRH output.
© 2022 The Authors.
Conflict of interest statement
Nothing declared.
Figures
References
-
- McArdle C.A., Roberson M.S. Knobil and Neill's Physiology of Reproduction. Elsevier; 2015. Gonadotropes and gonadotropin-releasing hormone signaling; pp. 335–397. - DOI
Publication types
LinkOut - more resources
Full Text Sources
