Hyalocyte origin, structure, and imaging
- PMID: 36632192
- PMCID: PMC9831111
- DOI: 10.1080/17469899.2022.2100762
Hyalocyte origin, structure, and imaging
Abstract
Introduction: Hyalocytes have been recognized as resident tissue macrophages of the vitreous body since the mid-19th century. Despite this, knowledge about their origin, turnover, and dynamics is limited.
Areas covered: Historically, initial studies on the origin of hyalocytes used light and electron microscopy. Modern investigations across species including rodents and humans will be described. Novel imaging is now available to study human hyalocytes in vivo. The shared ontogeny with retinal microglia and their eventual interdependence as well as differences will be discussed.
Expert opinion: Owing to a common origin as myeloid cells, hyalocytes and retinal microglia have similarities, but hyalocytes appear to be distinct as resident macrophages of the vitreous body.
Keywords: Hyalocytes; Imaging; Macrophages; Monocytes; Origin; Turnover; Vitreous.
Figures






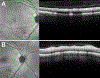
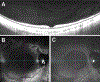
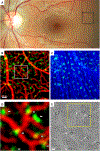

References
-
- Sebag J Vitreous: the resplendent enigma. British Journal of Ophthalmology. 2009;93:989–991. - PubMed
-
-
Sebag J Structure of the Vitreous. In: Sebag J, editor. The Vitreous: Structure, Function, and Pathobiology [Internet]. New York, NY: Springer; 1989. [cited 2022 Feb 23]. p. 35–58. Available from: 10.1007/978-1-4613-8908-8_4.
* This publication features phase contrast microscopy of unstained human hyalocytes in situ, as well as transmission electron microscopy of human hyalocytes.
-
-
- Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
