Sigma-1 receptor-regulated efferocytosis by infiltrating circulating macrophages/microglial cells protects against neuronal impairments and promotes functional recovery in cerebral ischemic stroke
- PMID: 36632219
- PMCID: PMC9830433
- DOI: 10.7150/thno.77088
Sigma-1 receptor-regulated efferocytosis by infiltrating circulating macrophages/microglial cells protects against neuronal impairments and promotes functional recovery in cerebral ischemic stroke
Abstract
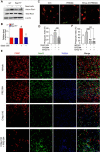
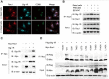
Background: Efferocytosis of apoptotic neurons by macrophages is essential for the resolution of inflammation and for neuronal protection from secondary damage. It is known that alteration of the Sigma-1 receptor (Sig-1R) is involved in the pathological development of some neurological diseases, including ischemic stroke. The present study aimed to investigate whether and how Sig-1R regulates the phagocytic activity of macrophages/microglia and its significance in neuroprotection and neurological function in stroke. Methods: The roles of Sig-1R in the efferocytosis activity of microglia/macrophages using bone marrow-derived macrophages (BMDMs) or using Sig-1R knockout mice subjected to transient middle artery occlusion (tMCAO)-induced stroke were investigated. The molecular mechanism of Sig-1R in the regulation of efferocytosis was also explored. Adoptive transfer of Sig-1R intact macrophages to recipient Sig-1R knockout mice with tMCAO was developed to observe its effect on apoptotic neuron clearance and stroke outcomes. Results: Depletion of Sig-1R greatly impaired the phagocytic activity of macrophages/microglia, accordingly with worsened brain damage and neurological defects in Sig-1R knockout mice subjected to tMCAO. Adoptive transfer of Sig-1R intact bone marrow-derived macrophages (BMDMs) to Sig-1R knockout mice restored the clearance activity of dead/dying neurons, reduced infarct area and neuroinflammation, and improved long-term functional recovery after cerebral ischemia. Mechanistically, Sig-1R-mediated efferocytosis was dependent on Rac1 activation in macrophages, and a few key sites of Rac1 in its binding pocket responsible for the interaction with Sig-1R were identified. Conclusion: Our data provide the first evidence of the pivotal role of Sig-1R in macrophage/microglia-mediated efferocytosis and elucidate a novel mechanism for the neuroprotection of Sig-1R in ischemic stroke.
Keywords: Efferocytosis; Ischemic stroke; Macrophage/microglia; Rac1; Sigma-1 receptor.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

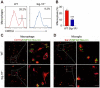

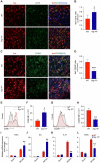
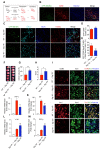
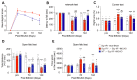
References
-
- Powers WJ. Acute ischemic stroke. N Engl J Med. 2020;383:252–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

