Bladder-colon chronic cross-sensitization involves neuro-glial pathways in male mice
- PMID: 36632316
- PMCID: PMC9827584
- DOI: 10.3748/wjg.v28.i48.6935
Bladder-colon chronic cross-sensitization involves neuro-glial pathways in male mice
Abstract
Background: Irritable bowel syndrome and bladder pain syndrome often overlap and are both characterized by visceral hypersensitivity. Since pelvic organs share common sensory pathways, it is likely that those syndromes involve a cross-sensitization of the bladder and the colon. The precise pathophysiology remains poorly understood.
Aim: To develop a model of chronic bladder-colon cross-sensitization and to investigate the mech-anisms involved.
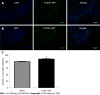
Methods: Chronic cross-organ visceral sensitization was obtained in C57BL/6 mice using ultrasound-guided intravesical injections of acetic acid under brief isoflurane anesthesia. Colorectal sensitivity was assessed in conscious mice by measuring intracolonic pressure during isobaric colorectal distensions. Myeloperoxidase, used as a marker of colorectal inflammation, was measured in the colon, and colorectal permeability was measured using chambers. c-Fos protein expression, used as a marker of neuronal activation, was assessed in the spinal cord (L6-S1 level) using immunohistochemistry. Green fluorescent protein on the fractalkine receptor-positive mice were used to identify and count microglia cells in the L6-S1 dorsal horn of the spinal cord. The expression of NK1 receptors and MAPK-p38 were quantified in the spinal cord using western blot.
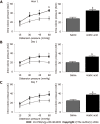
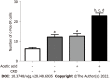
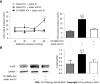
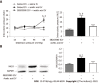
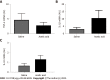
Results: Visceral hypersensitivity to colorectal distension was observed after the intravesical injection of acetic acid vs saline (P < 0.0001). This effect started 1 h post-injection and lasted up to 7 d post-injection. No increased permeability or inflammation was shown in the bladder or colon 7 d post-injection. Visceral hypersensitivity was associated with the increased expression of c-Fos protein in the spinal cord (P < 0.0001). In green fluorescent protein on the fractalkine receptor-positive mice, intravesical acetic acid injection resulted in an increased number of microglia cells in the L6-S1 dorsal horn of the spinal cord (P < 0.0001). NK1 receptor and MAPK-p38 levels were increased in the spinal cord up to 7 d after injection (P = 0.007 and 0.023 respectively). Colorectal sensitization was prevented by intrathecal or intracerebroventricular injections of minocycline, a microglia inhibitor, by intracerebroventricular injection of CP-99994 dihydrochloride, a NK1 antagonist, and by intracerebroventricular injection of SB203580, a MAPK-p38 inhibitor.
Conclusion: We describe a new model of cross-organ visceral sensitization between the bladder and the colon in mice. Intravesical injections of acetic acid induced a long-lasting colorectal hypersensitivity to distension, mediated by neuroglial interactions, MAPK-p38 phosphorylation and the NK1 receptor.
Keywords: Cross-organ sensitization; MAPK-p38; Microglia; NK1 receptor; Pain; Visceral hypersensitivity.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: None of the authors have any conflicts of interest.
Figures


Similar articles
-
Involvement of acid sensing ion channel (ASIC)-3 in an acute urinary bladder-colon cross sensitization model in rodent.Front Pain Res (Lausanne). 2023 Mar 8;4:1083514. doi: 10.3389/fpain.2023.1083514. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 36969917 Free PMC article.
-
Analgesic effect of minocycline in rat model of inflammation-induced visceral pain.Eur J Pharmacol. 2014 Mar 15;727:87-98. doi: 10.1016/j.ejphar.2014.01.026. Epub 2014 Jan 28. Eur J Pharmacol. 2014. PMID: 24485889 Free PMC article.
-
Acute sacral nerve stimulation reduces visceral mechanosensitivity in a cross-organ sensitization model.Neurogastroenterol Motil. 2017 Apr;29(4). doi: 10.1111/nmo.12987. Epub 2016 Nov 7. Neurogastroenterol Motil. 2017. PMID: 27997083
-
Spinal microglia: A potential target in the treatment of chronic visceral pain.J Chin Med Assoc. 2014 Jan;77(1):3-9. doi: 10.1016/j.jcma.2013.08.008. Epub 2013 Oct 1. J Chin Med Assoc. 2014. PMID: 24090601 Review.
-
Cross-talk and sensitization of bladder afferent nerves.Neurourol Urodyn. 2010;29(1):77-81. doi: 10.1002/nau.20817. Neurourol Urodyn. 2010. PMID: 20025032 Free PMC article. Review.
Cited by
-
Sex Differences in Visceral Pain and Comorbidities: Clinical Outcomes, Preclinical Models, and Cellular and Molecular Mechanisms.Cells. 2024 May 14;13(10):834. doi: 10.3390/cells13100834. Cells. 2024. PMID: 38786056 Free PMC article. Review.
-
Molecular Mechanisms and Pathways in Visceral Pain.Cells. 2025 Jul 25;14(15):1146. doi: 10.3390/cells14151146. Cells. 2025. PMID: 40801578 Free PMC article. Review.
-
"Sentinel or accomplice": gut microbiota and microglia crosstalk in disorders of gut-brain interaction.Protein Cell. 2023 Oct 25;14(10):726-742. doi: 10.1093/procel/pwad020. Protein Cell. 2023. PMID: 37074139 Free PMC article. Review.
References
-
- Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–57. - PubMed
-
- Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358–1363. - PubMed
-
- Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158:1262–1273.e3. - PubMed
-
- Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141–145. - PMC - PubMed
-
- Mathieu N. [Somatic comorbidities in irritable bowel syndrome: fibromyalgia, chronic fatigue syndrome, and interstitial cystitis] Gastroenterol Clin Biol. 2009;33 Suppl 1:S17–S25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

