Integrating real-world data to assess cardiac ablation device outcomes in a multicenter study using the OMOP common data model for regulatory decisions: implementation and evaluation
- PMID: 36632328
- PMCID: PMC9831049
- DOI: 10.1093/jamiaopen/ooac108
Integrating real-world data to assess cardiac ablation device outcomes in a multicenter study using the OMOP common data model for regulatory decisions: implementation and evaluation
Abstract
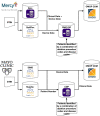
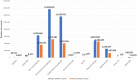
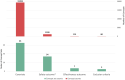
The objective of this study is to describe application of the Observational Medical Outcomes Partnership (OMOP) common data model (CDM) to support medical device real-world evaluation in a National Evaluation System for health Technology Coordinating Center (NESTcc) Test-Case involving 2 healthcare systems, Mercy Health and Mayo Clinic. CDM implementation was coordinated across 2 healthcare systems with multiple hospitals to aggregate both medical device data from supply chain databases and patient outcomes and covariates from electronic health record data. Several data quality assurance (QA) analyses were implemented on the OMOP CDM to validate the data extraction, transformation, and load (ETL) process. OMOP CDM-based data of relevant patient encounters were successfully established to support studies for FDA regulatory submissions. QA analyses verified that the data transformation was robust between data sources and OMOP CDM. Our efforts provided useful insights in real-world data integration using OMOP CDM for medical device evaluation coordinated across multiple healthcare systems.
Keywords: OMOP CDM; UDI; medical data standardization; medical device.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures
References
-
- Krucoff MW, Sedrakyan A, Normand SL.. Bridging nnmet medical device ecosystem needs with strategically coordinated registries networks. JAMA 2015; 314 (16): 1691–2. - PubMed
-
- Center for Devices and Radiological Health, US Food and Drug Administration. Strengthening our national system for medical device postmarket surveillance. 2013. http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProd.... Accessed July 29, 2022.
-
- US Food and Drug Administration. 2018-2020 Strategic Priorities. Center for Devices and Radiological Health. 2018. https://www.fda.gov/media/110478/download. Accessed July 29, 2022.
-
- Dhalluin T, Fakhiri S, Bouzillé G, et al.Role of real-world digital data for orthopedic implant automated surveillance: a systematic review. Expert Rev Med Devices 2021; 18 (8): 799–810. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
