Adjunctive minocycline for major depressive disorder: A sub-study exploring peripheral immune-inflammatory markers and associated treatment response
- PMID: 36632339
- PMCID: PMC9826878
- DOI: 10.1016/j.bbih.2022.100581
Adjunctive minocycline for major depressive disorder: A sub-study exploring peripheral immune-inflammatory markers and associated treatment response
Abstract
Background: Adjunctive minocycline shows promise in treating affective and psychotic disorders; however, the therapeutic mechanism remains unclear. Identifying relevant biomarkers may enhance the efficacy of novel adjunctive treatment candidates. We thus investigated the peripheral immune-inflammatory profile in a randomized controlled trial (RCT) of minocycline in major depressive disorder (MDD).
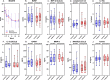
Methods: This sub-study investigated serum samples from a RCT evaluating minocycline (200 mg/day, 12 weeks) in addition to treatment as usual for MDD (ACTRN12612000283875). Of the original sample (N = 71), serum assays were conducted in 47 participants (placebo n = 24; minocycline n = 23) targeting an array of 46 immune-inflammatory analytes including cytokines, chemokines, and acute-phase reactants. General estimating equations (GEE) were used to assess whether analyte concentration at baseline (effect modification) and change in analytes (change association) influenced change in Montgomery-Åsberg Depression Rating Scale (MADRS) score over time. The Benjamini-Hochberg approach was applied when adjusting for false discovery rates (FDR).
Results: GEE models revealed several interaction effects. After adjusting for FDR several change association-models survived correction. However, no such models remained significant for effect modification. Three-way group × time × marker interactions were significant for complement C3 (B = -10.46, 95%CI [-16.832, -4.095], q = 0.019) and IL-1Ra (B = -9.008, 95%CI [-15.26, -2.751], q = 0.036). Two-way group × biomarker interactions were significant for ICAM-1/CD54 (B = -0.387, 95%CI [-0.513, -0.26], q < 0.001) and IL-8/CXCL8 (B = -4.586, 95%CI [-7.698, -1.475], q = 0.036) indicating that increases in the serum concentration of these analytes were associated with an improvement in MADRS scores in the minocycline group (compared with placebo).
Conclusions: Change in complement C3, IL-1Ra, IL-8/CXCL8, and ICAM-1 may be associated with greater change in depressive scores following adjunctive minocycline treatment in MDD. Further investigations are needed to assess the utility of these biomarkers.
Keywords: Biomarkers; Depression; Inflammation; Minocycline; Neuroscience; Psychiatry.
© 2023 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Bai S., Guo W., Feng Y., Deng H., Li G., Nie H., Guo G., Yu H., Ma Y., Wang J., Chen S., Jing J., Yang J., Tang Y., Tang Z. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatry. 2020;91:21–32. - PubMed
-
- Bauer O., Milenkovic V.M., Hilbert S., Sarubin N., Weigl J., Bahr L.M., Wetter T.C., Heckel B., Wetzel C.H., Rupprecht R., Nothdurfter C. Association of chemokine (C-C motif) receptor 5 and ligand 5 with recovery from major depressive disorder and related neurocognitive impairment. Neuroimmunomodulation. 2020;27:152–162. - PMC - PubMed
-
- Berk M., Wadee A.A., Kuschke R.H., O'Neill-Kerr A. Acute phase proteins in major depression. J. Psychosom. Res. 1997;43:529–534. - PubMed
-
- Berk M., Walker A.J., Nierenberg A.A. Biomarker-guided anti-inflammatory therapies: from promise to reality check. JAMA Psychiatr. 2019;76:779–780. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

