A novel drug-like water-soluble small molecule Focal Adhesion Kinase (FAK) activator promotes intestinal mucosal healing
- PMID: 36632414
- PMCID: PMC9827036
- DOI: 10.1016/j.crphar.2022.100147
A novel drug-like water-soluble small molecule Focal Adhesion Kinase (FAK) activator promotes intestinal mucosal healing
Abstract
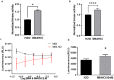
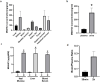
Non-steroidal anti-inflammatory drugs (NSAIDs) injure the proximal and distal gut by different mechanisms. While many drugs reduce gastrointestinal injury, no drug directly stimulates mucosal wound healing. Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, induces epithelial sheet migration. We synthesized and evaluated a water-soluble FAK-activating small molecule, M64HCl, with drug-like properties. Monolayer wound closure and Western blots measured migration and FAK phosphorylation in Caco-2 cells, in vitro kinase assays established FAK activation, and pharmacologic tests assessed drug-like properties. 30 mg/kg/day M64HCl was administered in two murine small intestine injury models for 4 days. M64HCl (0.1-1000 nM) dose-dependently increased Caco-2 FAK-Tyr 397 phosphorylation, without activating Pyk2 and accelerated Caco-2 monolayer wound closure. M64HCl dose-responsively activates the FAK kinase domain vs. the non-salt M64, increasing the Vmax of ATP-binding. Pharmacologic tests suggested M64HCl has drug-like properties and is enterally absorbed. M64HCl 25 mg/kg/day continuous infusion promoted healing of ischemic jejunal ulcers and indomethacin-induced small intestinal injury in C57Bl/6 mice. M64HCl-treated mice exhibited smaller ulcers 4 days after ischemic ulcer induction or indomethacin injury. Renal histology and plasma creatinine were normal. Mild hepatic inflammatory changes and ALT elevation were similar among M64HCl-treated mice and controls. M64HCl was concentrated in kidney and gastrointestinal mucosa and functional nephrectomy studies suggested predominantly urinary excretion. Little toxicity was observed in vitro or in single-dose mouse toxicity studies until >1000x higher than effective concentrations. M64HCl, a water-soluble FAK activator, promotes epithelial restitution and intestinal mucosal healing and may be useful to treat gut mucosal injury.
Keywords: Focal adhesion kinase; Mucosal healing; Non-steroidal anti-inflammatory drugs; Small intestine; Ulcer.
© 2022 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:The 10.13039/100007237University of North Dakota and the 10.13039/100007249University of Minnesota have filed two patent applications addressing the use of small molecule FAK activators in promoting mucosal healing. Drs. Basson and Gurvich and Gallardo-Macias are named as inventors in at least one of each of these patents. The authors have no other potential competing interests to declare.
Figures









Similar articles
-
Sustained intestinal epithelial monolayer wound closure after transient application of a FAK-activating small molecule.PLoS One. 2024 Aug 16;19(8):e0304010. doi: 10.1371/journal.pone.0304010. eCollection 2024. PLoS One. 2024. PMID: 39150901 Free PMC article.
-
M64HCl, a focal adhesion kinase activator, promotes intestinal mucosal healing in rats.BMC Gastroenterol. 2025 May 8;25(1):347. doi: 10.1186/s12876-025-03937-5. BMC Gastroenterol. 2025. PMID: 40340802 Free PMC article.
-
Small molecule FAK activator promotes human intestinal epithelial monolayer wound closure and mouse ulcer healing.Sci Rep. 2019 Oct 11;9(1):14669. doi: 10.1038/s41598-019-51183-z. Sci Rep. 2019. PMID: 31604999 Free PMC article.
-
Gut homeostasis, injury, and healing: New therapeutic targets.World J Gastroenterol. 2022 May 7;28(17):1725-1750. doi: 10.3748/wjg.v28.i17.1725. World J Gastroenterol. 2022. PMID: 35633906 Free PMC article. Review.
-
Progress in researches about focal adhesion kinase in gastrointestinal tract.World J Gastroenterol. 2009 Dec 21;15(47):5916-23. doi: 10.3748/wjg.15.5916. World J Gastroenterol. 2009. PMID: 20014455 Free PMC article. Review.
Cited by
-
Sustained intestinal epithelial monolayer wound closure after transient application of a FAK-activating small molecule.PLoS One. 2024 Aug 16;19(8):e0304010. doi: 10.1371/journal.pone.0304010. eCollection 2024. PLoS One. 2024. PMID: 39150901 Free PMC article.
-
Pathophysiology updates: gastroduodenal injury and repair mechanisms.Curr Opin Gastroenterol. 2023 Nov 1;39(6):512-516. doi: 10.1097/MOG.0000000000000973. Epub 2023 Aug 29. Curr Opin Gastroenterol. 2023. PMID: 37678191 Free PMC article. Review.
-
M64HCl, a focal adhesion kinase activator, promotes intestinal mucosal healing in rats.BMC Gastroenterol. 2025 May 8;25(1):347. doi: 10.1186/s12876-025-03937-5. BMC Gastroenterol. 2025. PMID: 40340802 Free PMC article.
-
Focal Adhesion Kinase and Colony Stimulating Factors: Intestinal Homeostasis and Innate Immunity Crosstalk.Cells. 2024 Jul 11;13(14):1178. doi: 10.3390/cells13141178. Cells. 2024. PMID: 39056760 Free PMC article. Review.
References
-
- Acebrón I., Righetto R.D., Schoenherr C., de Buhr S., Redondo P., Culley J., Rodríguez C.F., Daday C., Biyani N., Llorca O., Byron A., Chami M., Gräter F., Boskovic J., Frame M.C., Stahlberg H., Lietha D. Structural basis of Focal Adhesion Kinase activation on lipid membranes. EMBO J. 2020;39(19) - PMC - PubMed
-
- Ashton G.H., Morton J.P., Myant K., Phesse T.J., Ridgway R.A., Marsh V., Wilkins J.A., Athineos D., Muncan V., Kemp R., Neufeld K., Clevers H., Brunton V., Winton D.J., Wang X., Sears R.C., Clarke A.R., Frame M.C., Sansom O.J. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell. 2010;19(2):259–269. - PMC - PubMed
-
- Basson M.D. Hierarchies of healing in gut mucosal injury. J. Physiol. Pharmacol. 2017;68(6):789–795. - PubMed
-
- Basson M.D., Sanders M.A., Gomez R., Hatfield J., Vanderheide R., Thamilselvan V., Zhang J., Walsh M.F. Focal adhesion kinase protein levels in gut epithelial motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291(3):G491–G499. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

