Characterisation of functional deficits induced by AAV overexpression of alpha-synuclein in rats
- PMID: 36632447
- PMCID: PMC9827042
- DOI: 10.1016/j.crneur.2022.100065
Characterisation of functional deficits induced by AAV overexpression of alpha-synuclein in rats
Abstract
Background: In the last decades different preclinical animal models of Parkinson's disease (PD) have been generated, aiming to mimic the progressive neuronal loss of midbrain dopaminergic (DA) cells as well as motor and non-motor impairment. Among all the available models, AAV-based models of human alpha-synuclein (h-aSYN) overexpression are promising tools for investigation of disease progression and therapeutic interventions.
Objectives: The goal with this work was to characterise the impairment in motor and non-motor domains following nigrostriatal overexpression of h-aSYN and correlate the behavioural deficits with histological assessment of associated pathology.
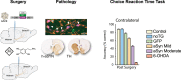
Methods: Intranigral injection of an AAV9 expressing h-aSYN was compared with untreated animals, 6-OHDA and AAV9 expressing either no transgene or GFP. The animals were assessed on a series of simple and complex behavioural tasks probing motor and non-motor domains. Post-mortem neuropathology was analysed using immunohistochemical methods.
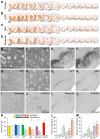
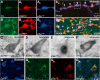
Results: Overexpression of h-aSYN led to progressive degeneration of DA neurons of the SN and axonal terminals in the striatum (STR). We observed extensive nigral and striatal pathology, resembling that of human PD brain, as well as the development of stable progressive deficit in simple motor tasks and in non-motor domains such as deficits in motivation and lateralised neglect.
Conclusions: In the present work we characterized a rat model of PD that closely resembles human PD pathology at the histological and behavioural level. The correlation of cell loss with behavioural performance enables the selection of rats which can be used in neuroprotective or neurorestorative therapies.
Keywords: AAV; Alpha-synuclein; Choice reaction time; Parkinson's disease; Visuo-spatial neglect.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Alarcon-Aris D., Pavia-Collado R., Miquel-Rio L., Coppola-Segovia V., Ferres-Coy A., Ruiz-Bronchal E., Galofre M., Paz V., Campa L., Revilla R., Montefeltro A., Kordower J.H., Vila M., Artigas F., Bortolozzi A. Anti-alpha-synuclein ASO delivered to monoamine neurons prevents alpha-synuclein accumulation in a Parkinson's disease-like mouse model and in monkeys. EBioMedicine. 2020;59 - PMC - PubMed
-
- Albert K., Voutilainen M.H., Domanskyi A., Piepponen T.P., Ahola S., Tuominen R.K., Richie C., Harvey B.K., Airavaara M. Downregulation of tyrosine hydroxylase phenotype after AAV injection above substantia nigra: caution in experimental models of Parkinson's disease. J. Neurosci. Res. 2019;97:346–361. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

