1,25-Dihydroxyvitamin D Deficiency Accelerates Aging-related Osteoarthritis via Downregulation of Sirt1 in Mice
- PMID: 36632467
- PMCID: PMC9830508
- DOI: 10.7150/ijbs.78785
1,25-Dihydroxyvitamin D Deficiency Accelerates Aging-related Osteoarthritis via Downregulation of Sirt1 in Mice
Abstract
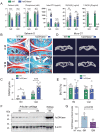
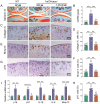
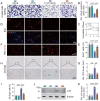
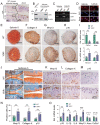
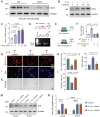
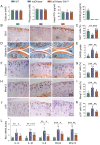
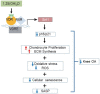
Emerging observational data suggest that vitamin D deficiency is associated with the onset and progression of knee osteoarthritis (OA). However, the relationship between vitamin D level and OA and the role of vitamin D supplementation in the prevention of knee OA are controversial. To address these issues, we analyzed the articular cartilage phenotype of 6- and 12-month-old wild-type and 1α(OH)ase-/- mice and found that 1,25(OH)2D deficiency accelerated the development of age-related spontaneous knee OA, including cartilage surface destruction, cartilage erosion, proteoglycan loss and cytopenia, increased OARSI score, collagen X and Mmp13 positive chondrocytes, and increased chondrocyte senescence with senescence-associated secretory phenotype (SASP). 1,25(OH)2D3 supplementation rescued all knee OA phenotypes of 1α(OH)ase-/- mice in vivo, and 1,25(OH)2D3 rescued IL-1β-induced chondrocyte OA phenotypes in vitro, including decreased chondrocyte proliferation and cartilage matrix protein synthesis, and increased oxidative stress and cell senescence. We also demonstrated that VDR was expressed in mouse articular chondrocytes, and that VDR knockout mice exhibited knee OA phenotypes. Furthermore, we demonstrated that the down-regulation of Sirt1 in articular chondrocytes of 1α(OH)ase-/- mice was corrected by supplementing 1,25(OH)2D3 or overexpression of Sirt1 in mesenchymal stem cells (MSCs) and 1,25(OH)2D3 up-regulated Sirt1 through VDR mediated transcription. Finally, we demonstrated that overexpression of Sirt1 in MSCs rescued knee OA phenotypes in 1α(OH)ase-/- mice. Thus, we conclude that 1,25(OH)2D3, via VDR-mediated gene transcription, plays a key role in preventing the onset of aging-related knee OA in mouse models by up-regulating Sirt1, an aging-related gene that promotes articular chondrocyte proliferation and extracellular matrix protein synthesis, and inhibits senescence and SASP.
Keywords: Sirt1; Vitamin D deficiency; osteoarthritis; vitamin D receptor; vitamin D supplementation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. - PubMed
-
- Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology (Oxford, England) 2013;52:1323–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

