MiRNA regulated therapeutic potential of the stromal vascular fraction: Current clinical applications - A systematic review
- PMID: 36632616
- PMCID: PMC9817091
- DOI: 10.1016/j.ncrna.2022.12.003
MiRNA regulated therapeutic potential of the stromal vascular fraction: Current clinical applications - A systematic review
Abstract
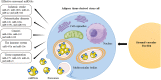
Introduction: The stromal vascular fraction (SVF) is a heterogeneous population of cells that, interacting with each other, can affect the processes of regeneration, angiogenesis, and immunomodulation. Over the past 20 years, there has been a trend towards an increase in the number of clinical studies on the therapeutic use of SVF. MicroRNAs (miRNAs) are also important regulators of cellular function and they have been shown to be involved in SVF cellular component function. The purpose of this study was to analyze existing clinical studies on the therapeutic use of SVF including the role of miRNAs in the regulation of the function of the cellular component of SVF as an anti-inflammatory, pro-angiogenic and cell differentiation activity.
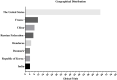
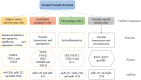
Methods: The search strategy was to use material from the clinicaltrials.gov website, which focused on the key term "Stromal vascular fraction", and the inclusion and exclusion criteria were divided into two stages.
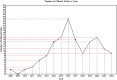
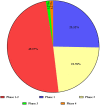
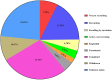
Results: By August 2022, there were 149 registered clinical trials. Most studies belong to either Phase 1-2 (49.37%), Phase 1 (25.32%) or Phase 2 (22.78%). Most of them focused in the fields of traumatology, neurology/neurosurgery, endocrinology, vascular surgery, and immunology. However, only 8 clinical trials had published results. All of clinical trials have similar preparation methods and 8 clinical trials have positive results with no serious adverse effects.
Conclusions: There appears to be a wide potential for the clinical use of SVF without reports of serious side effects. Many preclinical and clinical studies are currently underway on the use of SVF, and their future results will help to further explore their therapeutic potential. Nevertheless, there are not many studies on the role of miRNAs in the SVF microenvironment; however, this topic is very important for further study of the clinical application of SVF, including safety, in various human diseases.
Keywords: Clinical trial; Epidemiology; Stromal vascular fraction; Therapy; miRNA.
© 2022 The Authors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

