Can the bone marrow harvest volume be reduced safely in hematopoietic stem cell transplantation with pediatric sibling donors?
- PMID: 36632685
- PMCID: PMC10063592
- DOI: 10.5045/br.2023.2022167
Can the bone marrow harvest volume be reduced safely in hematopoietic stem cell transplantation with pediatric sibling donors?
Abstract
Background: Reduced harvest volumes in pediatric donors appear to have the potential to reduce donor-associated risks while maintaining engraftment in recipients; however, the allowable harvest volume reduction remains undefined.
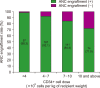
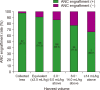
Methods: We retrospectively analyzed the data pairs of 553 bone marrow (BM) harvests from pediatric (age at harvest <18 yr) sibling donors and clinical outcomes of 553 pediatric (age at infusion <14 yr) transplant-naïve recipients to assess the optimal BM harvest volume needed from pediatric donors to obtain the desired CD34+ cell count (≥3.0×106 cells per kg of recipient weight), and to study its impact on the clinical outcomes of transplantation in pediatric recipients.
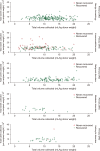
Results: The minimum desired CD34+ cell count of ≥3.0×106 per kg of recipient weight was achieved for 506 (95.3%) of donor-recipient pairs. The median CD34+ cell yield was 6.4×106 per kg of recipient weight (range, 1.2‒33.8×106) in donors younger than 5 years old at harvest, 4.7×106 (range, 0.3‒28.5×106) in donors aged 5‒10 years and 2.1×106 (range, 0.3‒11.3×106) in donors older than 10 years (P<0.001).
Conclusion: The infused CD34+ cell dose (×106 cells/kg of recipient weight) had no impact on GRFS; however, a CD34+ cell dose of >7×106 cells/kg of recipient weight did not improve hematopoietic recovery.
Keywords: Harvest volume; Marrow transplant; Pediatric donors.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Behfar M, Faghihi-Kashani S, Hosseini AS, Ghavamzadeh A, Hamidieh AA. Long-term safety of short-term administration of filgrastim (rhG-CSF) and leukophresis procedure in healthy children: application of peripheral blood stem cell collection in pediatric donors. Biol Blood Marrow Transplant. 2018;24:866–70. doi: 10.1016/j.bbmt.2017.12.786. - DOI - PubMed
-
- Styczynski J, Balduzzi A, Gil L, et al. Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Trans-plantation Pediatric Diseases Working Party study. Blood. 2012;119:2935–42. doi: 10.1182/blood-2011-04-349688. - DOI - PubMed
LinkOut - more resources
Full Text Sources

