A combined physiologically-based pharmacokinetic and quantitative systems pharmacology model for modeling amyloid aggregation in Alzheimer's disease
- PMID: 36632701
- PMCID: PMC10088087
- DOI: 10.1002/psp4.12912
A combined physiologically-based pharmacokinetic and quantitative systems pharmacology model for modeling amyloid aggregation in Alzheimer's disease
Abstract
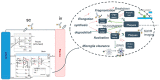
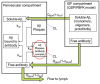
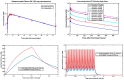
Antibody-mediated removal of aggregated β-amyloid (Aβ) is the current, most clinically advanced potential disease-modifying treatment approach for Alzheimer's disease. We describe a quantitative systems pharmacology (QSP) approach of the dynamics of Aβ monomers, oligomers, protofibrils, and plaque using a detailed microscopic model of Aβ40 and Aβ42 aggregation and clearance of aggregated Aβ by activated microglia cells, which is enhanced by the interaction of antibody-bound Aβ. The model allows for the prediction of Aβ positron emission tomography (PET) imaging load as measured by a standardized uptake value ratio. A physiology-based pharmacokinetic model is seamlessly integrated to describe target exposure of monoclonal antibodies and simulate dynamics of cerebrospinal fluid (CSF) and plasma biomarkers, including CSF Aβ42 and plasma Aβ42 /Aβ40 ratio biomarkers. Apolipoprotein E genotype is implemented as a difference in microglia clearance. By incorporating antibody-bound, plaque-mediated macrophage activation in the perivascular compartment, the model also predicts the incidence of amyloid-related imaging abnormalities with edema (ARIA-E). The QSP platform is calibrated with pharmacological and clinical information on aducanumab, bapineuzumab, crenezumab, gantenerumab, lecanemab, and solanezumab, predicting adequately the change in PET imaging measured amyloid load and the changes in the plasma Aβ42 /Aβ40 ratio while slightly overestimating the change in CSF Aβ42 . ARIA-E is well predicted for all antibodies except bapineuzumab. This QSP model could support the clinical trial design of different amyloid-modulating interventions, define optimal titration and maintenance schedules, and provide a first step to understand the variability of biomarker response in clinical practice.
© 2023 Eisai Inc. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Hugo Geerts, Mike Walker, Rachel Rose, Silke Bergeler, and Piet H. van der Graaf are employees of Certara. Edgar Schuck, Akihiko Koyama, Sanae Yasuda, Ziad Hussein, Larisa Reyderman, Chad Swanson, and Antonio Cabal are employees of Eisai Inc.
Figures




References
-
- Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306‐318. - PubMed
-
- Chai AB, Lam HHJ, Kockx M, Gelissen IC. Apolipoprotein E isoform‐dependent effects on the processing of Alzheimer's amyloid‐beta. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158980. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

