High rate of durable responses with undetectable minimal residual disease with front-line venetoclax and rituximab in young, fit patients with chronic lymphocytic leukemia and an adverse biological profile: results of the GIMEMA phase II LLC1518 - VERITAS study
- PMID: 36632738
- PMCID: PMC10388270
- DOI: 10.3324/haematol.2022.282116
High rate of durable responses with undetectable minimal residual disease with front-line venetoclax and rituximab in young, fit patients with chronic lymphocytic leukemia and an adverse biological profile: results of the GIMEMA phase II LLC1518 - VERITAS study
Abstract
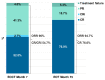
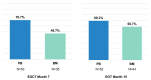
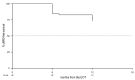
The GIMEMA phase II LLC1518 VERITAS trial investigated the efficacy and safety of front-line, fixed-duration venetoclax and rituximab (VenR) in combination in young (≤65 years), fit patients with chronic lymphocytic leukemia and unmutated IGHV and/or TP53 disruption. Treatment consisted of the venetoclax ramp-up, six monthly courses of the VenR combination, followed by six monthly courses of venetoclax as a single agent. A centralized assessment of minimal residual disease (MRD) was performed by allele-specific oligonucleotide polymerase chain reaction assay on the peripheral blood and bone marrow at the end of treatment (EOT) and during the follow-up. The primary endpoint was the complete remission rate at the EOT. Seventy-five patients were enrolled; the median age was 54 years (range, 38-65), 96% had unmutated IGHV, 12% had TP53 disruption, and 4% had mutated IGHV with TP53 disruption. The overall response rate at the EOT was 94.7%, with a complete remission rate of 76%. MRD was undetectable in the peripheral blood of 69.3% of patients and in the bone marrow of 58.7% of patients. The 12-month MRD-free survival in the 52 patients with undetectable MRD in the peripheral blood at the EOT was 73.1%. After a median follow-up of 20.8 months, no cases of disease progression were observed. Three patients had died, two due to COVID-19 and one due to tumor lysis syndrome. The first report of the VERITAS study shows that front-line VenR was associated with a high rate of complete remissions and durable response with undetectable MRD in young patients with chronic lymphocytic leukemia and unfavorable genetic characteristics. ClinicalTrials.gov identifier: NCT03455517.
Figures
Comment in
-
Choosing between family members is always a balancing act.Haematologica. 2023 Aug 1;108(8):1975-1978. doi: 10.3324/haematol.2022.282628. Haematologica. 2023. PMID: 36815386 Free PMC article. No abstract available.
References
-
- The Surveillance Epidemiology and End Results (SEER) Program of the National Cancer Institute. Cancer Stat Facts: Leukemia -Chronic Lymphocytic Leukemia (CLL). https://seer.cancer.gov/statfacts/html/clyl.html; 2021.
-
- Mauro FR, Foa R, Giannarelli D, et al. . Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood. 1999;94(2):448-454. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

