Circular RNA ARHGAP5 inhibits cisplatin resistance in cervical squamous cell carcinoma by interacting with AUF1
- PMID: 36632741
- PMCID: PMC10067438
- DOI: 10.1111/cas.15723
Circular RNA ARHGAP5 inhibits cisplatin resistance in cervical squamous cell carcinoma by interacting with AUF1
Abstract
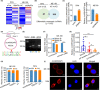
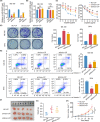
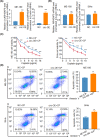
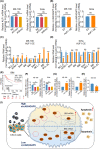
Cervical squamous cell carcinoma (CSCC) is one of the leading causes of cancer death in women worldwide. Patients with advanced cervical carcinoma always have a poor prognosis once resistant to cisplatin due to the lack of effective treatment. It is urgent to investigate the molecular mechanisms of cisplatin resistance. Circular RNAs (circRNAs) are known to exert their regulatory functions in a series of malignancies. However, their effects on CSCC remain to be elucidated. Here, we found that cytoplasmic circARHGAP5, derived from second and third exons of the ARHGAP5 gene, was downregulated in cisplatin-resistant tissues compared with normal cervix tissues and untreated cervical cancer tissues. In addition, experiments from overexpression/knockdown cell lines revealed that circARHGAP5 could inhibit cisplatin-mediated cell apoptosis in CSCC cells both in vitro and in vivo. Mechanistically, circARHGAP5 interacted with AU-rich element RNA-binding protein (AUF1) directly. Overexpression of AUF1 could also inhibit cell apoptosis mediated by cisplatin. Furthermore, we detected the potential targets of AUF1 related to the apoptotic pathway and found that bcl-2-like protein 11 (BIM) was not only negatively regulated by AUF1 but positively regulated by circARHGAP5, which indicated that BIM mRNA might be degraded by AUF1 and thereby inhibited tumor cell apoptosis. Collectively, our data indicated that circARHGAP5 directly bound to AUF1 and prevented AUF1 from interacting with BIM mRNA, thereby playing a pivotal role in cisplatin resistance in CSCC. Our study provides insights into overcoming cancer resistance to cisplatin treatment.
Keywords: AUF1; BIM; CSCC; cervical cancer; circARHGAP5.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures



Similar articles
-
AUF1 ligand circPCNX reduces cell proliferation by competing with p21 mRNA to increase p21 production.Nucleic Acids Res. 2021 Feb 22;49(3):1631-1646. doi: 10.1093/nar/gkaa1246. Nucleic Acids Res. 2021. PMID: 33444453 Free PMC article.
-
Upregulation of AUF1 is involved in the proliferation of esophageal squamous cell carcinoma through GCH1.Int J Oncol. 2016 Nov;49(5):2001-2010. doi: 10.3892/ijo.2016.3713. Epub 2016 Sep 28. Int J Oncol. 2016. PMID: 27826622
-
The telomere/telomerase binding factor PinX1 regulates paclitaxel sensitivity depending on spindle assembly checkpoint in human cervical squamous cell carcinomas.Cancer Lett. 2014 Oct 10;353(1):104-14. doi: 10.1016/j.canlet.2014.07.012. Epub 2014 Jul 18. Cancer Lett. 2014. PMID: 25045845
-
Circular RNA Circ_0002762 promotes cell migration and invasion in cervical squamous cell carcinoma via activating RelA/nuclear factor kappa B (Nf-kB) signalling pathway.RNA Biol. 2025 Dec;22(1):1-13. doi: 10.1080/15476286.2025.2478539. Epub 2025 Mar 24. RNA Biol. 2025. PMID: 40083243 Free PMC article.
-
Physiological networks and disease functions of RNA-binding protein AUF1.Wiley Interdiscip Rev RNA. 2014 Jul-Aug;5(4):549-64. doi: 10.1002/wrna.1230. Epub 2014 Mar 28. Wiley Interdiscip Rev RNA. 2014. PMID: 24687816 Review.
Cited by
-
CircRNA (circ)_0007823 Contributes to Triple-Negative Breast Cancer Progression and Cisplatin Resistance via the miR-182-5p/FOXO1 Pathway.Biochem Genet. 2025 Apr;63(2):1330-1342. doi: 10.1007/s10528-024-10783-9. Epub 2024 Apr 1. Biochem Genet. 2025. PMID: 38557813
-
Circular RNA EIF3I promotes papillary thyroid cancer progression by interacting with AUF1 to increase Cyclin D1 production.Oncogene. 2023 Oct;42(43):3206-3218. doi: 10.1038/s41388-023-02830-3. Epub 2023 Sep 11. Oncogene. 2023. PMID: 37697064
-
Cisplatin-Based Combination Therapy for Enhanced Cancer Treatment.Curr Drug Targets. 2024;25(7):473-491. doi: 10.2174/0113894501294182240401060343. Curr Drug Targets. 2024. PMID: 38591210 Review.
-
Circular RNAs in gynecologic cancers: mechanisms and implications for chemotherapy resistance.Front Pharmacol. 2023 Jun 8;14:1194719. doi: 10.3389/fphar.2023.1194719. eCollection 2023. Front Pharmacol. 2023. PMID: 37361215 Free PMC article. Review.
-
Mechanisms and therapeutic implications of gene expression regulation by circRNA-protein interactions in cancer.Commun Biol. 2025 Jan 17;8(1):77. doi: 10.1038/s42003-024-07383-z. Commun Biol. 2025. PMID: 39825074 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Small W, Bacon MA, Bajaj A, et al. Cervical cancer: a global health crisis. Cancer. 2017;123(13):2404‐2412. - PubMed
-
- Das M. WHO launches strategy to accelerate elimination of cervical cancer. Lancet Oncol. 2021;22(1):20‐21. - PubMed
-
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169‐182. - PubMed
-
- Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340‐1348. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

